Application of biomarker of psoriasis vulgaris blood plasma in target drugs
A type of psoriasis vulgaris, biomarker technology, applied in the field of biomarker screening, can solve the problems of low resolution and low sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
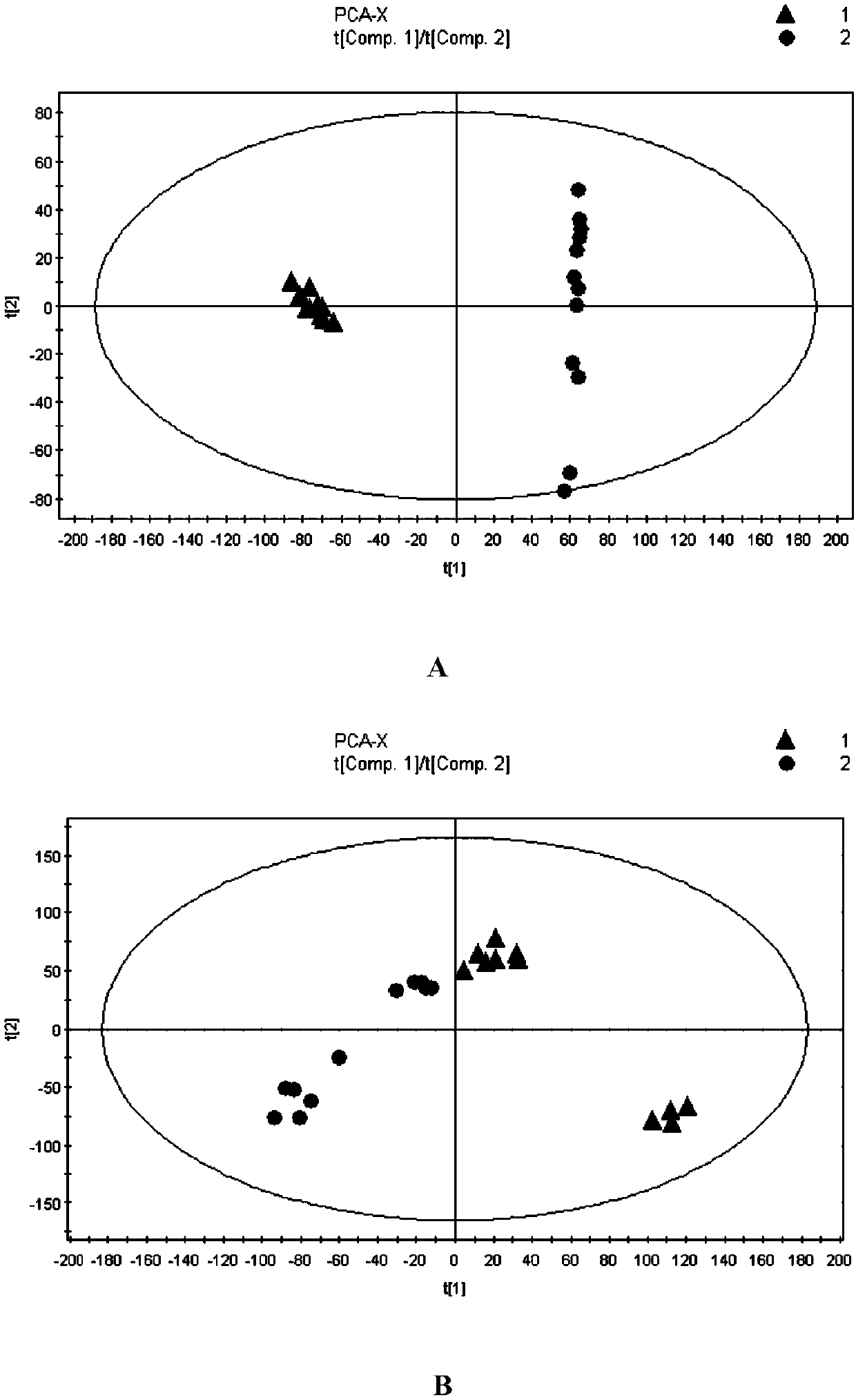
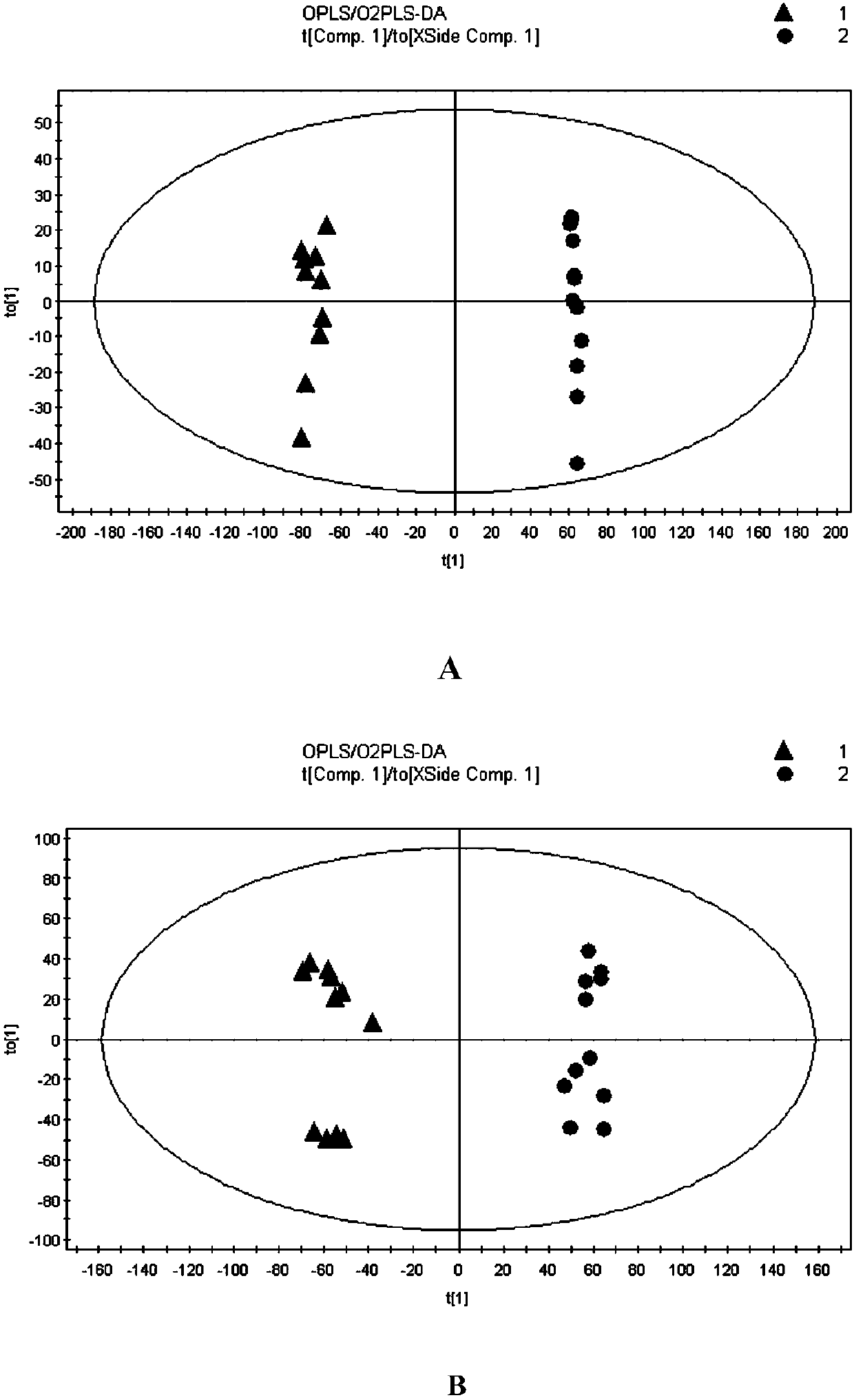
Image
Examples
Embodiment 1
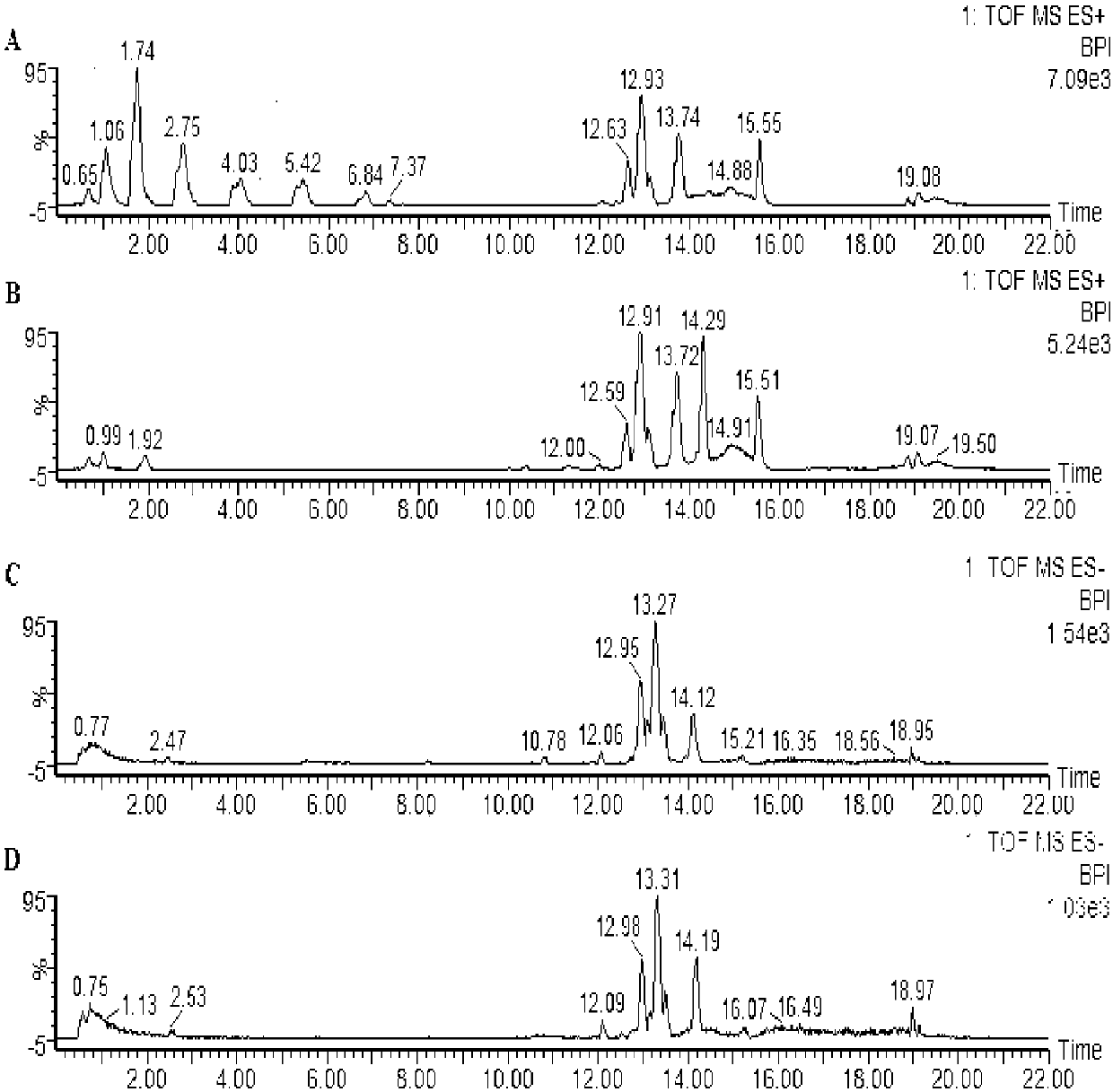
[0030] (1) Establishment of imiquimod-induced mouse psoriasis model: 24 female BALB / c mice were adaptively fed for 3 days and anesthetized with 4% chloral hydrate (0.1ml / 10g) intraperitoneally, and their backs were excised. Hair, forming an exposed area with a size of about 2cm×3cm, was randomly divided into 2 groups, 12 in each group, which were blank group and model group. Blank group: smear 62.5mg of Vaseline ointment on the bare back of the mice every day, and at the same time, gavage with normal saline, 0.2mL / time / day, for 7 consecutive days. Model group: smear 5% IMQ cream 62.5mg on the bare back of the mice at regular intervals every day, and at the same time, gavage with normal saline, 0.2mL / time / day, for 7 consecutive days.
[0031] (2) Collection and processing of mouse plasma samples: the mice in each group were drawn on the 8th day, and after the mice were anesthetized by intraperitoneal injection of 4% chloral hydrate solution (0.1ml / 10g), the blood was taken from...
Embodiment 2
[0036] Except step (2), all the other steps are identical with embodiment 1.
[0037] (2) Collection and processing of mouse plasma samples: the mice in each group were drawn on the 8th day, and after the mice were anesthetized by intraperitoneal injection of 4% chloral hydrate solution (0.1ml / 10g), the blood was taken from the eyeballs of the mice, and released In the centrifuge tube containing EDTA, immediately after blood collection, gently invert and mix 5-6 times, let it stand for 2 hours, centrifuge (4°C, 4000r / min, 10min), take the supernatant and store it in a -80°C refrigerator until Measurement. Before analysis, put the mouse plasma sample at room temperature for about 20 minutes until completely thawed, draw 200 μL of plasma, add 200 μL of acetonitrile to it, vortex for 30 s, centrifuge (12000r / min, 10min, 4°C), and take 750 μL of supernatant , blow dry with nitrogen, reconstitute the residue with 100 μL of methanol-water (10:90, v / v), vortex for 30 s, centrifuge (...
Embodiment 3
[0039] Except step (2), all the other steps are identical with embodiment 1.
[0040] (2) Collection and processing of mouse plasma samples: the mice in each group were drawn on the 8th day, and after the mice were anesthetized by intraperitoneal injection of 4% chloral hydrate solution (0.1ml / 10g), the blood was taken from the eyeballs of the mice, and released In the centrifuge tube containing EDTA, immediately after blood collection, gently invert and mix 5-6 times, let it stand for 2 hours, centrifuge (4°C, 4000r / min, 10min), take the supernatant and store it in a -80°C refrigerator until Measurement. Before analysis, put the mouse plasma sample at room temperature for about 20 minutes until completely thawed, draw 200 μL of plasma, add 200 μL of methanol to it, vortex for 30 seconds, centrifuge (12000r / min, 10min, 4°C), and take 750 μL of supernatant , blow dry with nitrogen, redissolve the residue with 100 μL of acetonitrile-water (10:90, v / v), vortex for 30 s, centrifu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com