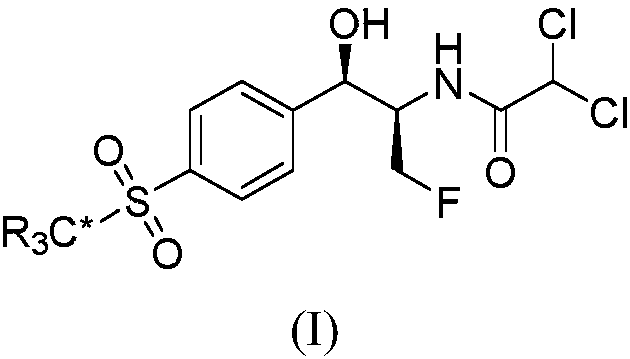
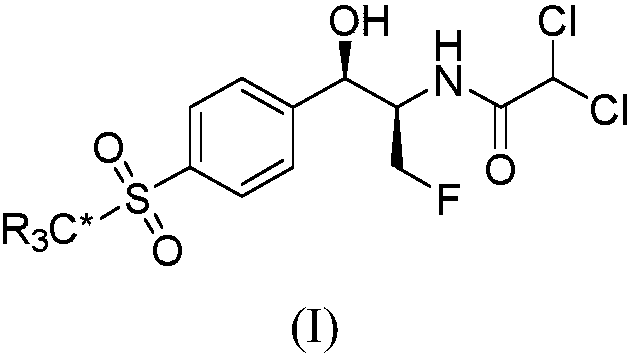
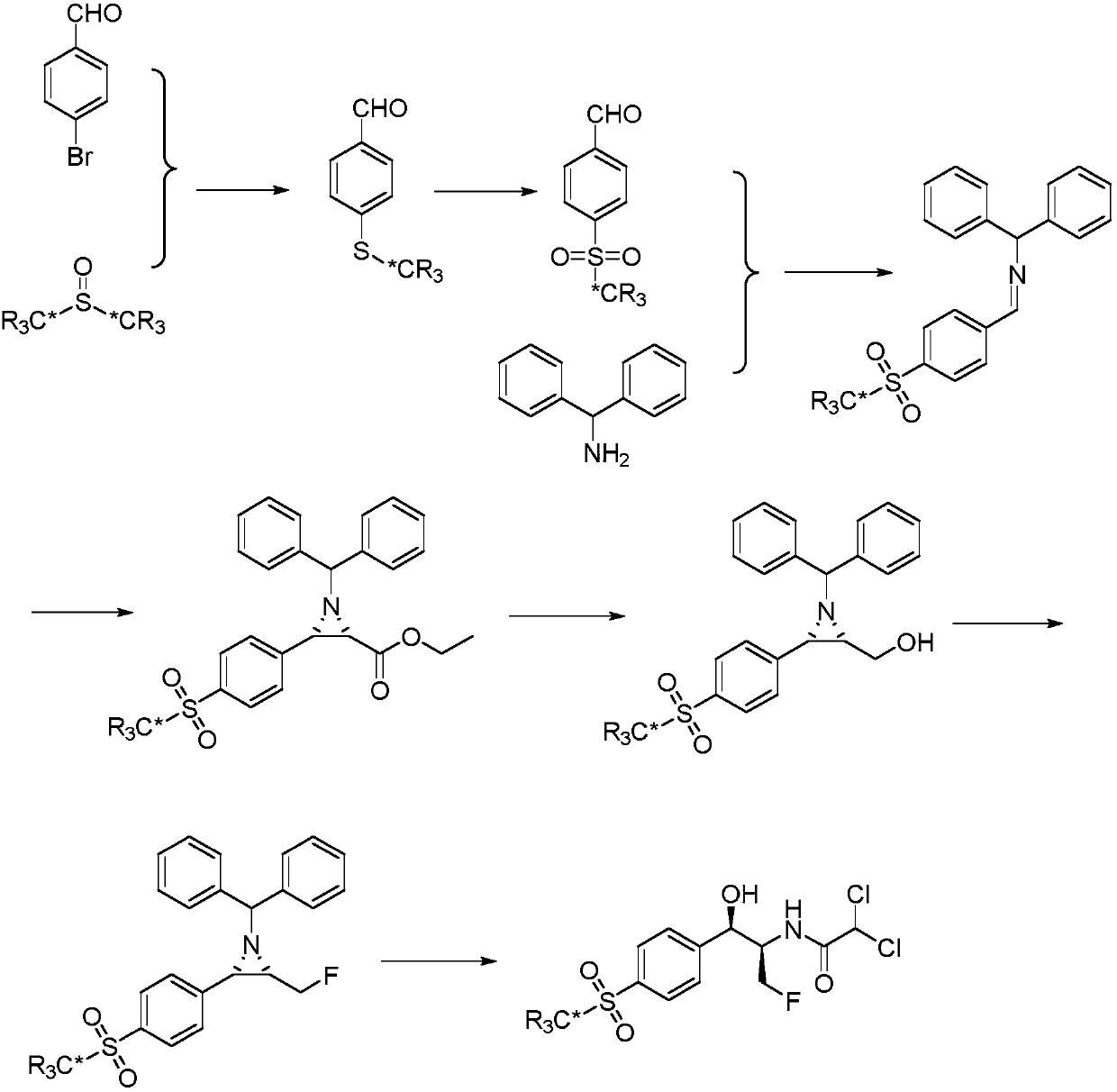
Synthetic method for stable isotope labeled florfenicol
A stable isotope and florfenicol technology, applied in the direction of isotope introduction of organic compounds, heterocyclic compound isotope introduction, acyclic/carbocyclic compound isotope introduction, etc., can solve the problem of low utilization rate and long route of stable isotope labeling intermediates , low total yield and other problems, to achieve the effect of simple synthesis steps, reduced production costs, and high product yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Embodiment 1: the synthetic method of deuterium-labeled 4-(methylmercapto) benzaldehyde
[0028]
[0029] In a sealed 25 mL glass tube, p-bromobenzaldehyde (184 mg, 1 mmol), cuprous iodide (190 mg, 1 mmol) and dry deuterated dimethyl sulfoxide (5 mL) were added. Filled with nitrogen, reacted at 130°C for 12-36h. After the reaction was completed, it was cooled to room temperature, and the catalyst was removed by filtration. The filtrate was extracted with ethyl acetate (3×20 mL), the organic phases were combined, washed with water (3×20 mL), washed with saturated sodium chloride solution (3×20 mL), and dried over anhydrous sodium sulfate. After filtration, the solvent was removed to obtain a crude product, and then deuterium-labeled 4-(methylmercapto)benzaldehyde (143 mg, 92%) was obtained as a pure product by column chromatography. H NMR (CDCl 3 , 600M) δppm 9.92(s, 1H), 7.77(d, 2H), 7.32(d, 2H); MS ESI+156[M+1].
Embodiment 2
[0030] Embodiment 2: the synthetic method of deuterium-labeled 4-thiamphenicol benzaldehyde
[0031]
[0032] In a 50mL three-necked flask, add 0.5M aqueous sodium hydroxide solution, add m-CPBA (380mg, 2.2mmol), stir well, add 4-(methylmercapto)benzaldehyde-methyl-D 3 (155mg, 1mmol), stirred at 0-5°C for 2 hours. Filter, extract the filtrate with ethyl acetate (3×20mL), combine the organic phases, wash with saturated sodium bicarbonate solution (3×20mL), water (3×20mL), and saturated sodium chloride solution (3×20mL) , adding anhydrous sodium sulfate to dry. The desiccant was removed by filtration, the solvent was distilled off under reduced pressure, and the residue was subjected to column chromatography to obtain deuterium-labeled 4-thiamphenicol benzaldehyde (187 mg, 96.2%). H NMR (CDCl 3 ,600M) δppm 9.88(s,1H), 7.81(d,2H), 7.36(d,2H); MS ESI+188[M+1].
Embodiment 3
[0033] Embodiment 3: The synthetic method of deuterium-labeled N-dianilino-1-(4-thiamphenicol phenyl) methylimine
[0034]
[0035] In a 100mL three-necked flask, add absolute ethanol (20mL), deuterated 4-thiamphenicol benzaldehyde (935mg, 5mmol), and diphenylmethylamine (920mg, 5mmol), react at 80°C for 2 hours, and cool to room temperature , the precipitated crystals were collected by filtration, washed with a small amount of cold ethanol, and dried under vacuum at 45° C. to obtain deuterium-labeled N-dianilino-1-(4-thiamphenylphenyl)methylimine (1.67 g, 95%). HNMR (CDCl 3 ,600M) δppm: 8.49(s,1H), 8.02(m,4H), 7.24~7.41(m,10H), 5.67(s,1H); MS ESI+353[M+1].
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com