Method for synthesizing trans-4-hydroxy-L-proline by virtue of escherichia coli
A technology of Escherichia coli and proline, applied in the fields of synthetic biology, medicinal chemistry, and microbial genetic engineering, can solve the problem of low HYP production, achieve rapid growth, increase production, and easy genetic manipulation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] Embodiment 1: Drawing of L-proline standard curve
[0057] The L-proline standard substance with a concentration of 1g / L was serially diluted to 250mg / L, 125mg / L, 75mg / L, 37.5mg / L, 18.75mg / L, 9.375mg / L, 4.687mg / L L, the concentration of 2.344mg / L and 1.172mg / L, with H 2 O is used as a blank control, and the absorbance values at 520 nm corresponding to different concentrations of L-proline are measured according to the ninhydrin method. Draw the standard curve, calculate the standard curve formula and R 2 value. Such as figure 2 As shown, the content of proline was calculated with the formula fitted by the standard curve.
Embodiment 2
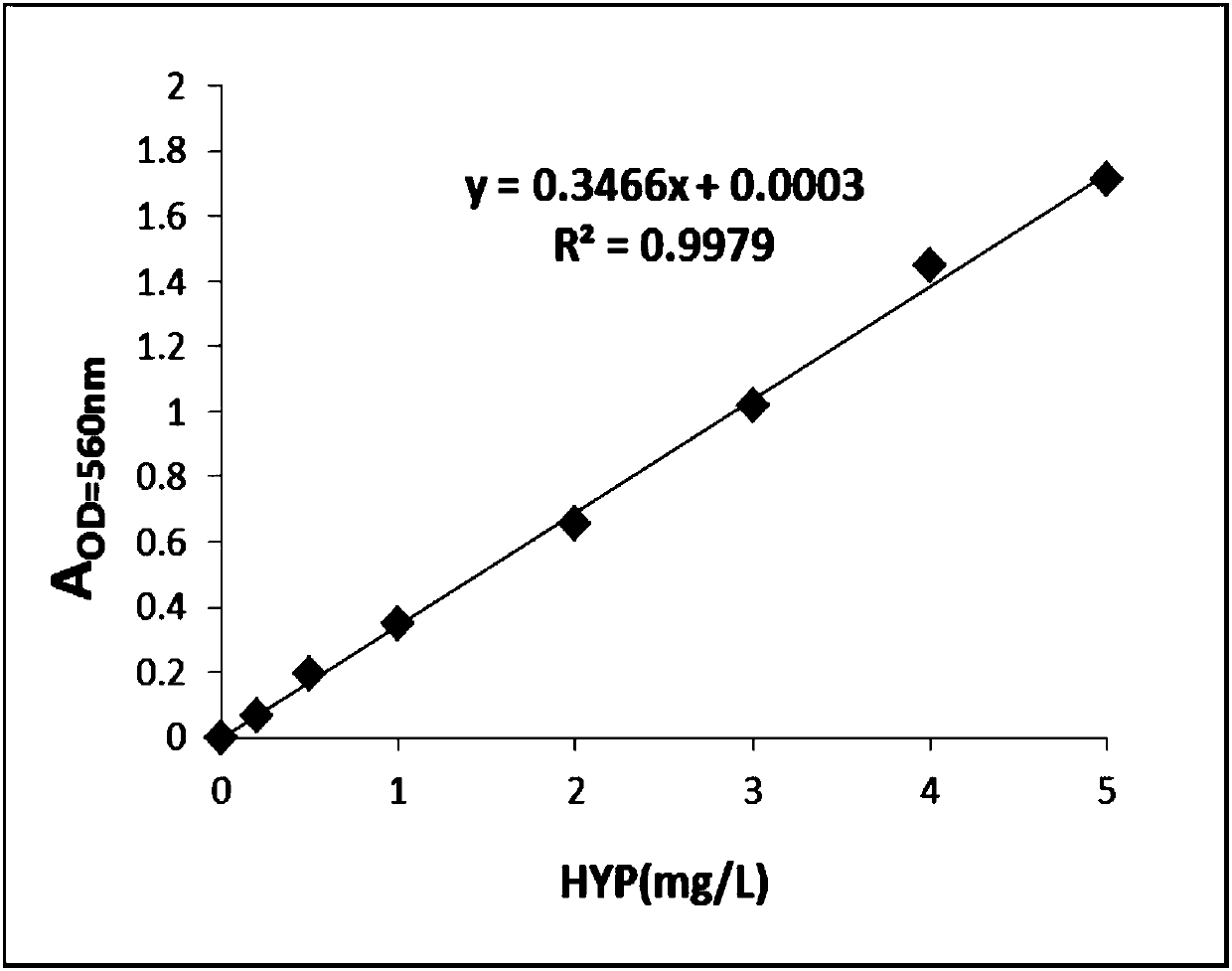
[0058] Embodiment 2: Drawing of trans-4-hydroxyl-L-proline standard curve
[0059]The trans-4-hydroxy-L-proline standard substance with a concentration of 1g / L was serially diluted to 0.2mg / L, 0.5mg / L, 1mg / L, 2mg / L, 3mg / L, 4mg / L, 5mg / L concentration, with H 2 O is used as a blank control, and the absorbance values at 560 nm corresponding to different concentrations of trans-4-hydroxyl-L-proline are measured according to the chloramine T method. Draw the standard curve, calculate the standard curve formula and R 2 value. Such as image 3 As shown, the content of trans-4-hydroxyl-L-proline was calculated by the formula fitted by the standard curve.
Embodiment 3
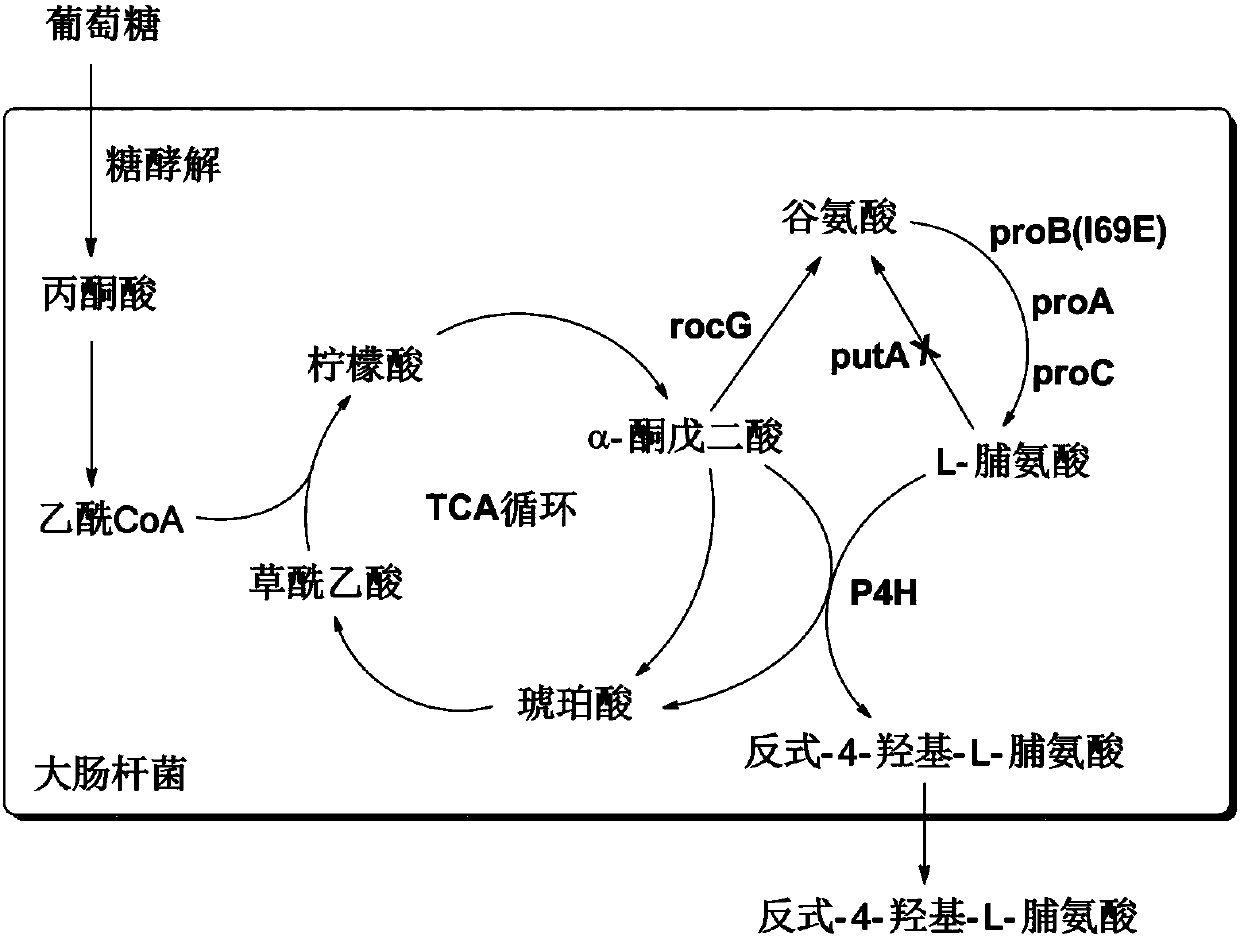
[0060] Embodiment 3: the construction of Escherichia coli MG1655 (ΔputA) host bacteria
[0061] Competent cells of Escherichia coli MG1655 were prepared with 10% glycerol, transformed into pKD46 plasmid, cultured on an ampicillin plate at 30°C overnight, and positive clones were screened. Then use positive clones to prepare competent cells: when the bacteria grow to OD 600nm When =0.2~0.3, add L-arabinose (30mM), induce for 1 hour at 30°C to express λ-red recombinant protein (exo, bet, gram), and harvest the bacteria to make MG1655 (pKD46) competent cells. Design primers with ~50bp homology arms upstream and downstream of the putA gene (Gene ID: 945600) coding region, the primers are:
[0062] putA-Ecoli-H1P1:GACCACGTTAAAGATGCCGGAGGAGGTTGTAACATCCTCCGGCTACCTGTAGCGATTGTGTAGGCTGGAG;
[0063] putA-Ecoli-H2P2:ACGATAACGTTAAGTTGCACCTTTCTGAACAACAGGAGTAATGGCATGGGAATTAGCCATGGTCC.
[0064] It is used to amplify the sequence between priming site 1 and 2 on pKD4, recover the amplified D...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com