Genetically engineered bacteria that degrade pet plastic
A hydrolase, Escherichia coli technology, applied in plastic recycling, bacteria, hydrolase and other directions, can solve the problems of slow growth of strains, fast restriction, effective application, difficult industrial application, etc., and achieve the effect of simple and efficient degradation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
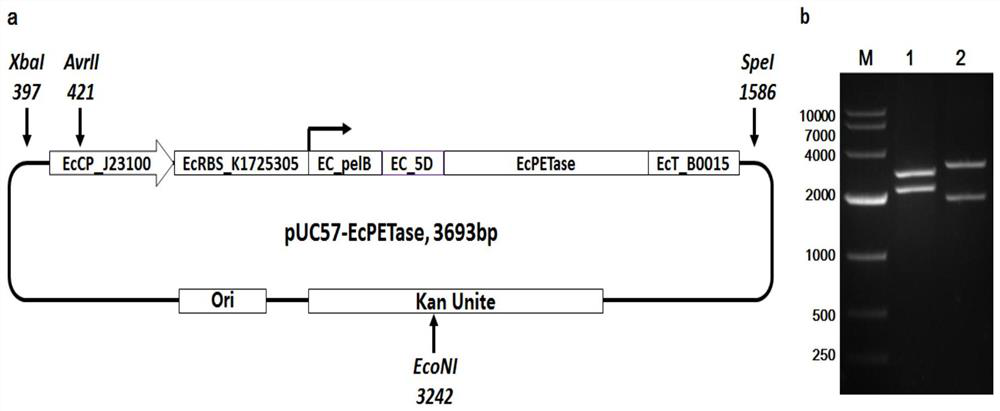
[0040] Embodiment 1, the DNA recombinant vector design, construction, preparation of Escherichia coli secreted type often expressing PET hydrolase (PETase)
[0041] Through artificial synthesis, Nanjing GenScript Biotechnology Co., Ltd. was entrusted to synthesize the PET hydrolase secreted regular expression unit in pUC57-EcPETase:
[0042] EcCP_J23100+EcRBS_K1725305+EC_pelB+Ec_5D+Ec_PETase+EcT_B0015 (Seq ID No. 1). This unit is composed of 6 sub-functional units, the specific design and the specific structure of each sub-unit are described as follows:
[0043] 1), EcCP_J23100: Escherichia coli constitutively expressed constant promoter, the nucleotide sequence is shown in the 1st to 35th positions of Seq ID No.1;
[0044] 2), EcRBS_K1725305: Escherichia coli protein translation RBS sequence, the nucleotide sequence is shown in the 36th to 62nd positions of Seq ID No.1;
[0045] 3), EC_pelB: Escherichia coli protein secretion expression signal peptide, codon optimization fo...
Embodiment 2
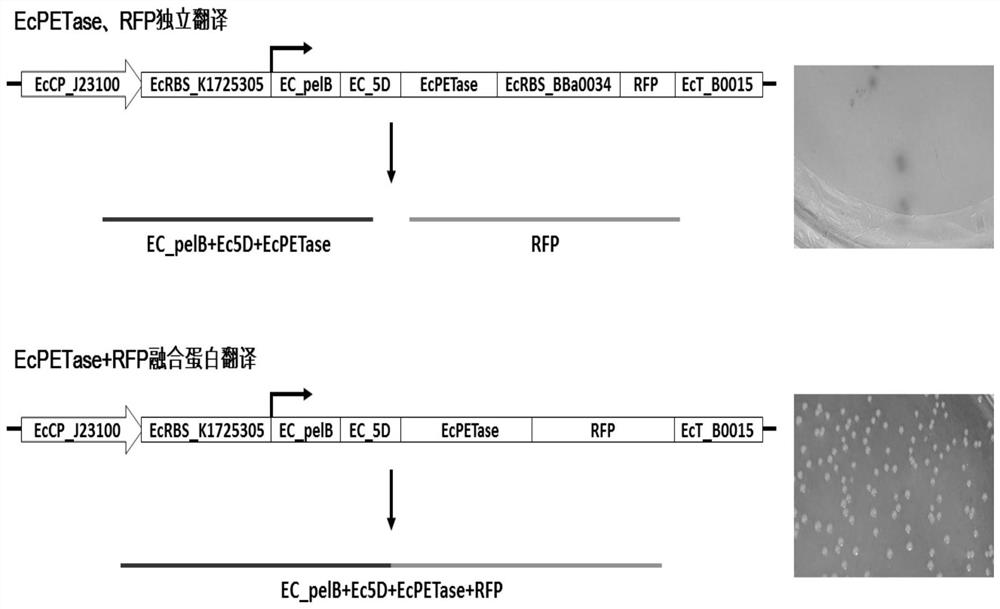
[0050] Embodiment 2, expression activity evaluation of pUC57-EcPETase recombinant DNA vector
[0051] Based on the constructed pUC57-EcPETase and the reported red fluorescent protein (RFP) reporter vector (pSB1C3-BBa_J04450), the RFP reporter gene was further introduced into the downstream of the PETase expression frame of the pUC57-EcPETase recombinant DNA plasmid by a synthetic method to obtain the same Two kinds of EcPETase+RFP co-expression vectors driven by the promoter (EcCP_J23100): 1) EcPETase and RFP have independent RBS sequences, which are transcribed simultaneously, and the translation products are two independent proteins (EcPETase, RFP); 2) EcPETase and RFP express The frame is fused, only one RBS sequence is used, and the translation product is one fusion protein (EcPETase-RFP fusion protein). The two EcPETase+RFP co-expression vectors were transformed into the expression strain E.coliBL21. After screening and identification, red recombinant colonies could be se...
Embodiment 3
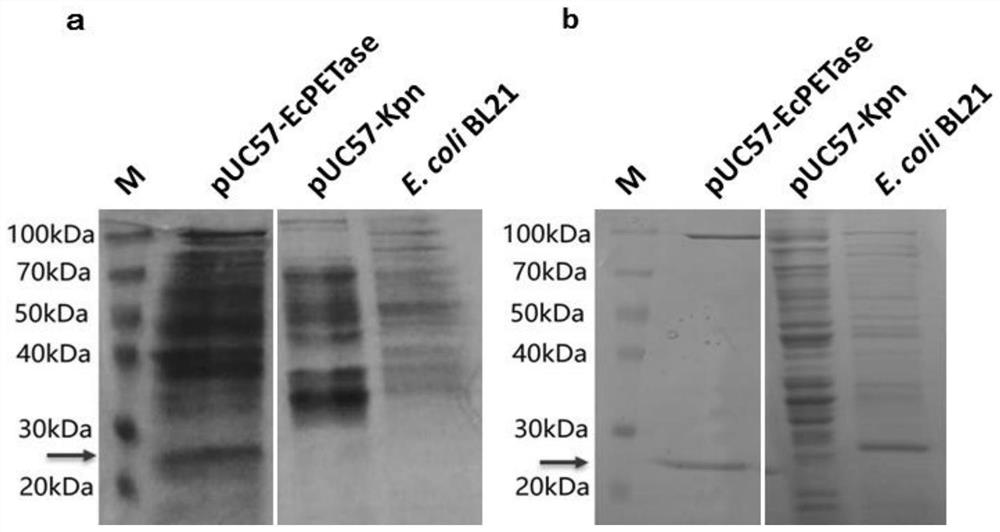
[0053] Example 3, pUC57-EcPETase Genetic Engineering Escherichia coli Strain Lipase Activity and PET Plastic Degradation Experiment
[0054] Referring to the pUC57-EcPETase genetically engineered Escherichia coli strain cultivation and sample preparation scheme in Example 2, the supernatant concentrated sample of the overnight culture product of the pUC57-EcPETase genetically engineered Escherichia coli strain was obtained, and pNPB was used as the substrate. Reference: "oshida S ,Hiraga K,Takehana T,Taniguchi I,Yamaji H,Maeda Y,Toyohara K,Miyamoto K,Kimura Y,Oda K.2016.A bacterium that degrades andassimilates poly(ethylene terephthalate).Science,351(6278):1196- 1199. ", test the EcPETase in the culture solution of pUC57-EcPETase genetically engineered Escherichia coli strain. The experimental results showed that the pUC57-EcPETase genetically engineered Escherichia coli strain culture fluid had significant lipase biological activity, and its pNPB hydrolysis efficiency was 10....
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com