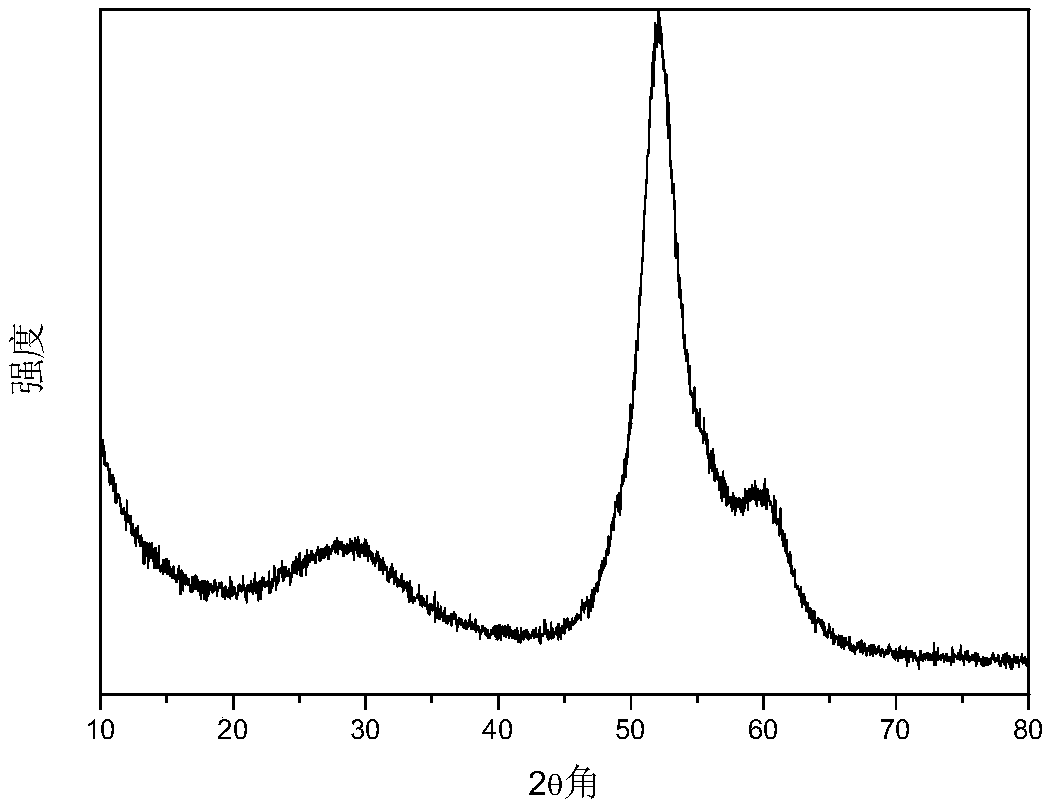
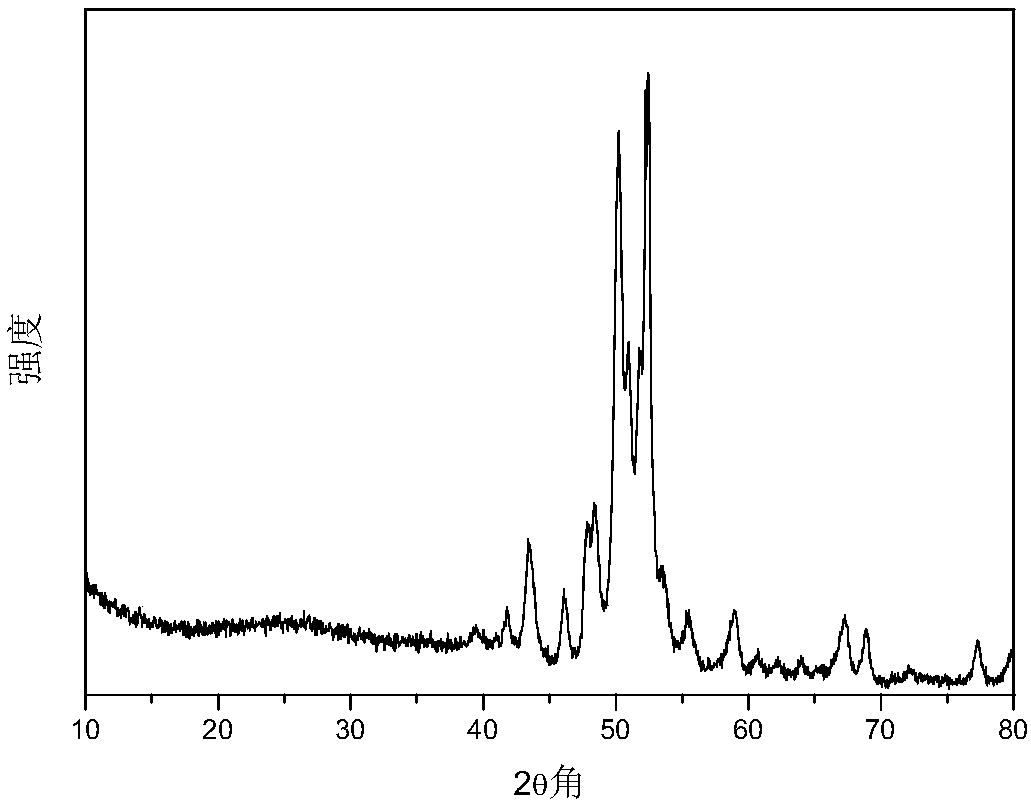
Porous carbon supported Fischer-Tropsch synthesis catalyst as well as preparation method and application thereof
A Fischer-Tropsch synthesis and catalyst technology, which can be used in chemical instruments and methods, preparation of liquid hydrocarbon mixtures, catalysts for physical/chemical processes, etc., and can solve problems such as high production costs, unsuitable for large-scale industrial production, and long preparation process routes.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0087] Take by weighing 44.7g terephthalic acid (H 2 BDC), 1179g N,N-dimethylformamide (DMF), 71.5g cobalt sulfate heptahydrate and 4.1g manganese sulfate, mixed, stirred until dissolved, transferred to a hydrothermal synthesis kettle, hydrothermally synthesized at 150°C for 36h, filtered , washing, and drying to obtain the CoMn-BDC organic carboxylic acid copolymer precursor (belonging to the 4th) kind of precursor), and its BET specific surface area is 331m 2 / g. Take by weighing potassium nitrate 1.5g and deionized water 44g to be mixed with solution, this solution is mixed with the prepared CoMn-BDC organic carboxylic acid copolymer precursor, dry, at 50% C 2 h 4 / 50%N 2 Carbonize at 600°C for 2h in airflow to obtain carbon-coated CoMn nanocomposites. The carbon-coated CoMn nanocomposite was kneaded with 48.0 g methylcellulose and extruded into strips with a diameter of 1 mm. 2 It was dried at 120°C in the atmosphere and calcined at 400°C to obtain a catalyst, marked ...
Embodiment 2
[0092] Weigh 25.2g of 2,5-dihydroxyterephthalic acid, 63.4g of cobalt acetate tetrahydrate, 27.5g of tetrahydrofuran, and 18.3g of deionized water, stir well until dissolved, transfer to a hydrothermal synthesis kettle, and crystallize at 110°C for 72h , the resulting product is filtered, washed, and dried to finally obtain a CPO-27-Co metal-organic framework material precursor (belonging to the fifth) precursor). Put the CPO-27-Co precursor at 20%C 2 h 6 Carbon-coated Co nanocomposites were obtained by carbonizing at 850°C for 1 h in 80% Ar gas flow. Carbon-coated Co nanocomposites were kneaded with 25.0 g of ethyl cellulose and extruded into strips with a diameter of 1 mm. 2 It was dried at 120°C in the atmosphere and calcined at 450°C to obtain a catalyst, which is marked as Exam-2. The mass composition of catalyst elements is: Co / C=24.5:100, and its texture properties, dispersion, reduction and wear index are listed in Table 1. The Fischer-Tropsch synthesis performance...
Embodiment 3
[0094] Weigh 24.7g cobalt nitrate hexahydrate, 5.18g 50% manganese nitrate solution, 0.01g platinum chloride, 50g starch, 8g ionized water (belonging to the 3rd kind of precursor), fully stir and mix to obtain a mixture of metal and carbonaceous substance Precursors, extruded into bars with a diameter of 1 mm, dried, and then heated at 20% C 3 h 6 Carbonization at 700° C. for 1 h in 80% Ar flow to obtain a catalyst, marked as Exam-3. The element mass composition of the catalyst is: Co / Mn / Pt / C=35.5:1.1:0.04:100, and its texture properties, dispersion degree, reduction degree and wear index are listed in Table 1.
[0095] The Fischer-Tropsch synthesis performance test was carried out in the same manner as in Example 1, and the results are listed in Table 2.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com