Transdermal preparation for treating oral mucositis caused by radiotherapy of tumor
A technology for oral mucositis and transdermal drug delivery, which is applied in the field of medicine and can solve problems such as poor efficacy, lack of ideal drugs for oral mucositis, and strong side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Example 1 Preparation of Gel Transdermal Drug Delivery Preparation
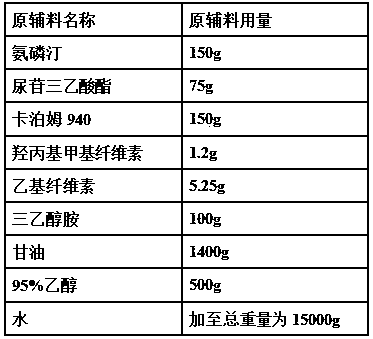
[0039]
[0040] Preparation:
[0041] (1) Take Carbomer 940 and add water to swell, add glycerin to grind and moisten, then add triethanolamine dropwise and grind to form a gel matrix;
[0042] (2) Take hydroxypropyl methylcellulose, ethyl cellulose and 95% ethanol to mix and moisten, add water to dissolve, add uridine triethyl
[0043] acid ester to make liquid dispersion system A;
[0044] (3) Take amifostine, dissolve it in water, and make liquid dispersion system B;
[0045] (4) Add liquid dispersion system A and liquid dispersion system B into the gel matrix, grind while adding, add water to a total weight of 15000 g, and stir evenly to obtain a gel transdermal drug delivery preparation.
Embodiment 2
[0046] Example 2 Preparation of Gel Transdermal Drug Delivery Preparation
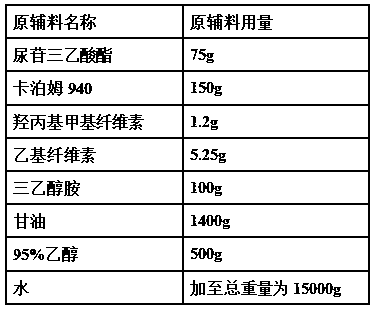
[0047]
[0048] Preparation:
[0049] (1) Take Carbomer 940 and add water to swell, add glycerin to grind and moisten, then add triethanolamine dropwise and grind to form a gel matrix;
[0050] (2) Take hydroxypropyl methylcellulose, ethyl cellulose and 95% ethanol to mix and moisten, add water to dissolve, add uridine triethyl
[0051] acid ester to make liquid dispersion system A;
[0052] (3) Take amifostine, dissolve it in water, and make liquid dispersion system B;
[0053] (4) Add liquid dispersion system A and liquid dispersion system B into the gel matrix, grind while adding, add water to a total weight of 10,000 g, and stir evenly to obtain a gel transdermal drug delivery preparation.
Embodiment 3
[0054] Example 3 Preparation of Gel Transdermal Drug Delivery Preparation
[0055]
[0056] Preparation:
[0057] (1) Take Carbomer 940 and add water to swell, add glycerin to grind and moisten, then add triethanolamine dropwise and grind to form a gel matrix;
[0058] (2) Take hydroxypropyl methylcellulose, ethyl cellulose and 95% ethanol to mix and moisten, add water to dissolve, add uridine triethyl
[0059] acid ester to make liquid dispersion system A;
[0060] (3) Take amifostine, dissolve it in water, and make liquid dispersion system B;
[0061] (4) Add liquid dispersion system A and liquid dispersion system B into the gel matrix, grind while adding, add water to a total weight of 15000 g, and stir evenly to obtain a gel transdermal drug delivery preparation.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com