Kit for determining heparin
A technology for heparin determination and kit, which is applied in biological tests, measuring devices, material inspection products, etc., can solve the problems of reducing the simplicity of the heparin determination kit, and achieve the effects of simple operation, high sensitivity, and cost reduction.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Composition and preparation method of embodiment 1 heparin assay kit
[0037] R1 reagent is mainly prepared from the following raw materials: porcine FXa concentration is 1.25U / ml, Tris is 50mM, BSA is 6.64mg / ml, polyethylene glycol-6000 is 10mg / ml and mannitol is 100mM, adjusted with 6M HCl pH to 8.4, lyophilized in 1ml / vial aliquots.
[0038] The R2 reagent was prepared from the following reagents: the chromogenic substrate Suc-Ile-Glu-(γ-Piperidyl)-Gly-Arg-pNA·HCl was 3.0 mg / ml, the acharan sulfate lyase was 1.0 U / ml, and Tris was 50mM, 0.02g / L dextran sulfate, 10mg / ml polyethylene glycol-6000 and 100mM mannitol, adjust the pH to 7.5 with 6M HCl, and freeze-dry in 1ml / bottle.
Embodiment 2
[0039] The assay method of embodiment 2 detection kits
[0040] (1) Reconstitute the R1 reagent and R2 reagent obtained in Example 1:
[0041] Reconstitute each bottle of R1 reagent with 5ml of distilled water, and each bottle of R2 reagent with 2ml of distilled water.
[0042] (2) Taking the operation of BioTek ELx800 microplate reader (end point method) as an example, set the analysis program according to the instrument instructions: the measurement wavelength is 405nm;
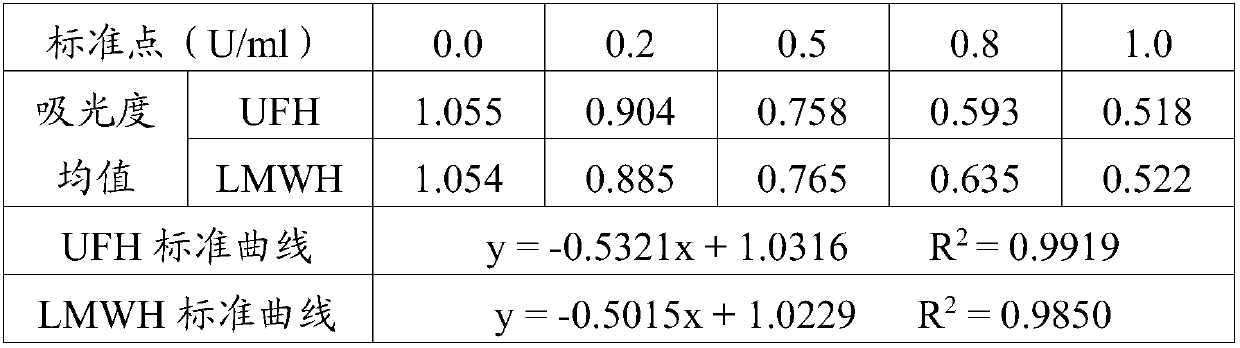
[0043] Firstly, dilute the heparin with known potency to 100U / ml heparin with normal saline. Take 10 μl of 100 U / ml heparin, add 990 μl of human plasma standard substance to dilute, and obtain 1.0 U / ml heparin standard substance. Continue to dilute the 1.0U / ml heparin standard with the human plasma standard, prepare 5 standard samples of 0.0, 0.2, 0.5, 0.8, 1.0U / ml heparin, and incubate at 37°C for 30 seconds. Take 50 μl of the diluted sample, add 50 μl of R2 reagent and incubate at 37°C for 60 seconds, ...
Embodiment 3
[0048] Embodiment 3 Analytical performance evaluation of the kit of the present invention
[0049] (1) Sensitivity
[0050] With the heparin standard product of 0.0U / ml as blank sample, use the test kit of the present invention obtained in Example 1, the detection method described in Example 2, repeat the measurement 20 times, and calculate the OD of this sample 405nm average and standard deviation (SD), the blank mean minus twice the standard deviation was substituted into the calibration curve equation to calculate the lowest detection limit, and the results are shown in Table 2.
[0051] Table 2 Minimum detection limit analysis
[0052]
[0053] The results in Table 2 show that the minimum detection limit of the kit of the present invention for detecting heparin can reach 0.02 U / ml, which is lower than that of the commercially available heparin detection kit (chromogenic substrate method, BIOPHEN Heparin kit of Aniara Company in France, article number: 221006) instruc...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com