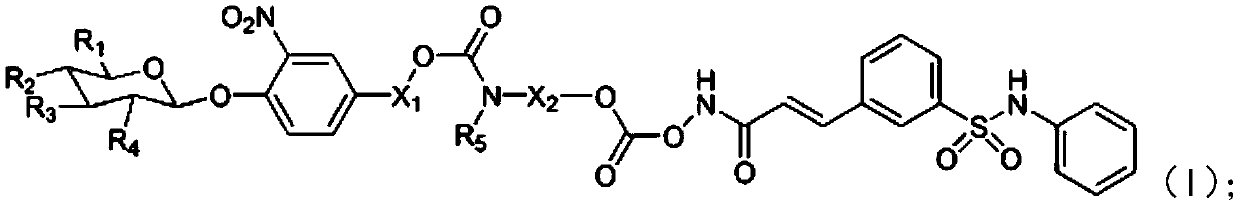
A kind of belinostat derivative based on acetic acid and its preparation method and application
A technology of belinostat and its derivatives, applied in the field of acetic acid-based belinostat derivatives and its preparation, to achieve the effects of reducing the burden of medication, controlling drug costs, and reducing the number of steps in the reaction process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
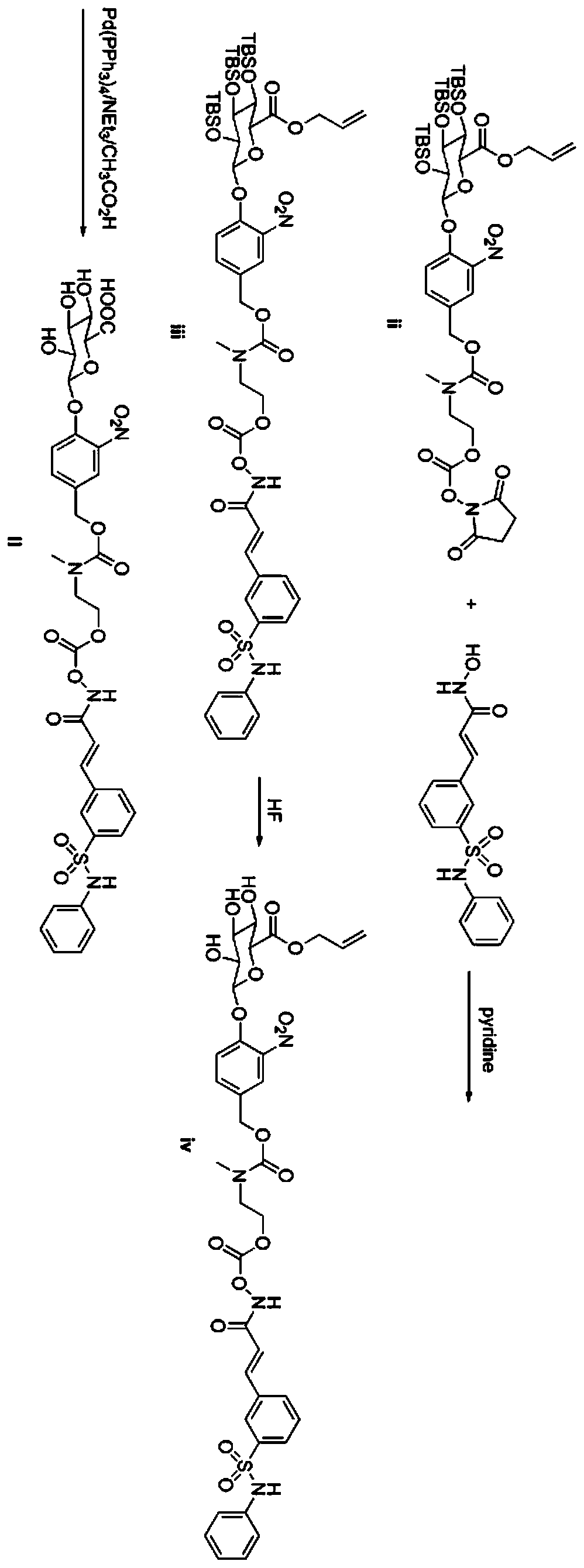
[0064] Example 1 O-{[N-methyl-N-4-(2,3,4-tri-O-tert-butyldimethylsilyl-6-allyl-β-D-glucopyranose Preparation of aldehyde acid-1-yl)-3-nitrobenzyloxycarbonyl]-2-aminoethyl}-formyl-belinostat (iii)
[0065] Bellinostat (469mg, 1.47mmol) was dissolved in 6.4mL of tetrahydrofuran, and the system was cooled to 0°C; then 1.6mL of pyridine was slowly added dropwise; the mixture was first stirred at 25°C for 5 minutes, and then added into formula (ii) Compound (1.3g, 1.34mmol); the reaction solution was kept at 25°C and stirred for 16 hours.
[0066] Then 20 mL of water was added to dilute and quench the reaction, and extracted with ethyl acetate (20 mL×2); the organic phases were combined, washed with 20 mL of saturated brine, dried over anhydrous sodium sulfate, filtered, and concentrated with a rotary evaporator. The residue was separated by preparative chromatography (petroleum ether: ethyl acetate = 3:1 to 1:1) to obtain a white solid, namely the following intermediate compound ...
Embodiment 2
[0068]Example 2 O-{[N-methyl-N-4-(6-allyl-β-D--pyranoglucuronic acid-1-yl)-3-nitrobenzyloxycarbonyl]-2- Preparation of aminoethyl}-formyl-belinostat (iv)
[0069] Compound iii (400mg, 0.34mmol) was dissolved in a mixed solvent of tetrahydrofuran (20mL) and acetonitrile (20mL);
[0070] Hydrofluoric acid (4.8mL, 40% in H 2 O) Dissolved in acetonitrile (15.2 mL) to prepare a solution, this solution was added to the solution containing compound iii at 0°C; the resulting reaction solution was stirred at 20°C for 2 days. The reaction liquid was concentrated to about 8 mL, and the following white solid iv (130 mg, 30.7%) was obtained by preparative HPLC separation.
[0071] Characterization of the product: 1 H NMR(400MHz,DMSO-d6):δ12.39(brs,1H),10.35(brs,1H),7.99(s,1H),7.89-7.86(m,2H),7.74(d,J=7.6Hz ,1H),7.68-7.58(m,3H),7.46(d,J=8.8Hz,1H),7.23(t,J=7.6Hz,2H),7.09(d,J=7.6Hz,2H),7.03 (t,J=7.6Hz,1H),6.60(d,J=16.0Hz,1H),5.93-5.85(m,1H),5.56-5.51(m,2H),5.34-5.28(m,3H), 5.18(d,J=10.8...
Embodiment 3
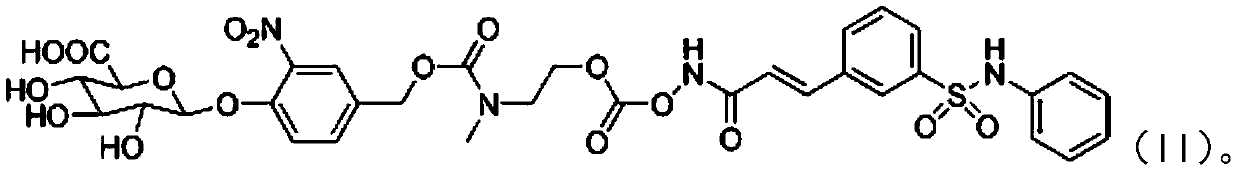
[0073] Example 3 O-{[N-methyl-N-4-(β-D-pyranoglucuron-1-yl)-3-nitrobenzyloxycarbonyl]-2-aminoethyl}-formyl - Preparation of Belinostat (II)
[0074] Compound iv (183mg, 0.22mmol) was dissolved in 10mL THF;
[0075] Dissolve 95 μL of triethylamine and 10 μL of acetic acid in 250 μL of tetrahydrofuran, and add the resulting solution to the tetrahydrofuran solution containing compound iv;
[0076] Pass argon for about 10 minutes, add a little tetrakistriphenylphosphopalladium, stir at room temperature for about 30 minutes until the raw material disappears, concentrate with a rotary evaporator, and separate the residue by preparative chromatography (acetonitrile: water = 20:1) to obtain the following white target: Product II (144 mg, 83%).
[0077] Characterization of the product: 1 H NMR (400MHz, DMSO-d 6 ):δ12.62(brs,1H),10.43(brs,1H),7.95-7.91(m,1H),7.65(s,1H),7.83-7.80(m,1H),7.52-7.46(m,1H ),7.48(d,J=16.0Hz,1H),7.19(m,2H),7.08-7.05(m,1H),6.95-6.92(m,5H),6.89(d,J=16.0Hz,1H...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com