Benzimidazole derivative, preparation method thereof and application of benzimidazole derivative to tumor resistance
A technology of benzimidazoles and derivatives, which is applied in its preparation method and its application in anti-tumor. In the field of benzimidazole derivatives, it can solve the problems of limited application and limited types of topoisomerase inhibitors.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
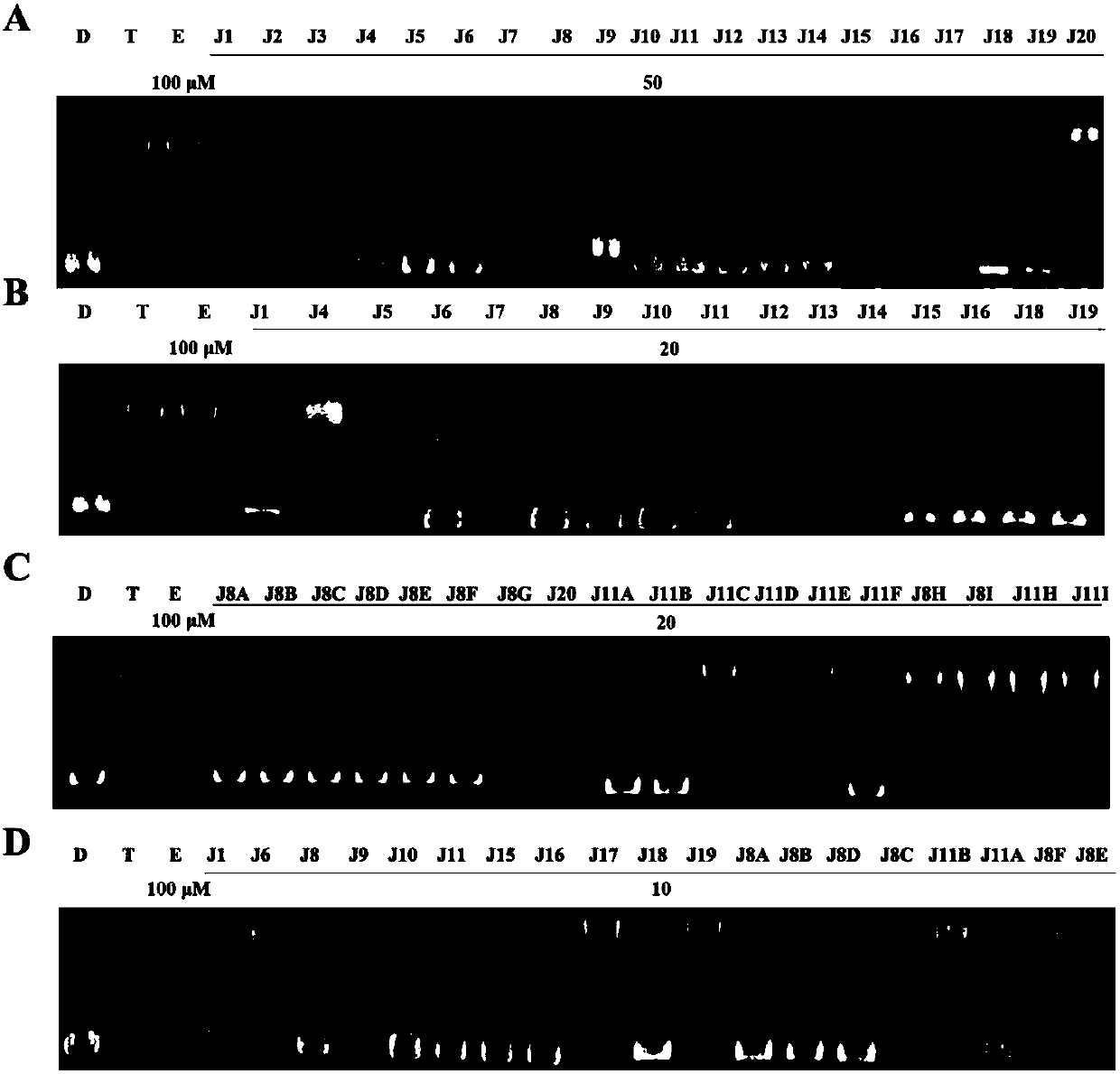
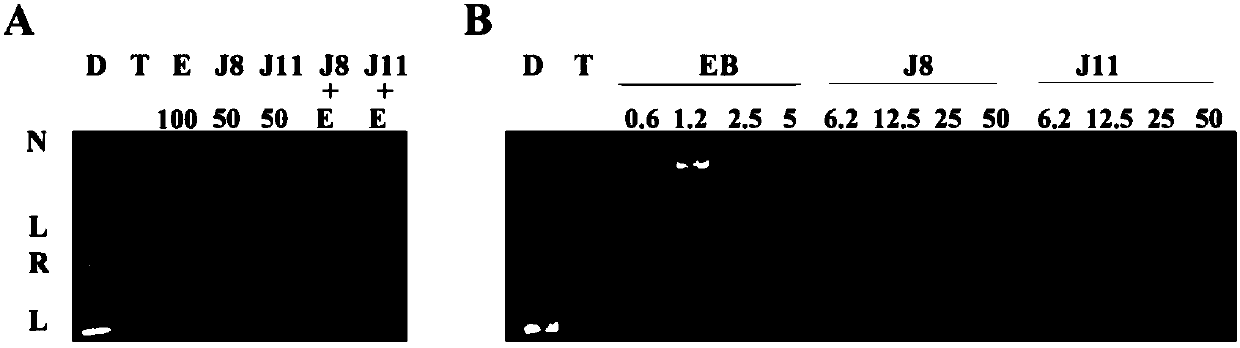
Image
Examples
preparation example Construction
[0063] The present invention also provides a kind of preparation method of benzimidazole derivatives, comprising the following steps:
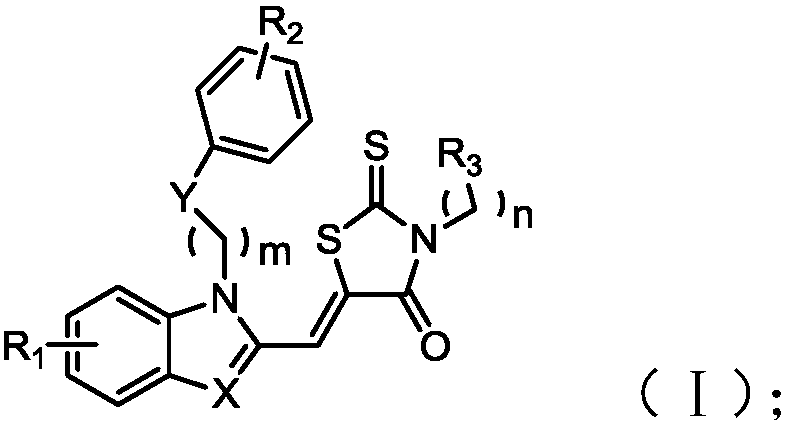
[0064] performing a first condensation reaction on rhodanine or its derivatives with a first compound having a structure represented by formula (II), to obtain a benzimidazole derivative having a structure represented by formula (I);
[0065]
[0066] Among them, R 1 and R 2 Independently selected from one of hydrogen, halogen, alkyl, alkoxy, haloalkyl, nitro and nitrile; preferably independently selected from hydrogen, halogen, alkyl with 1 to 5 carbon atoms, carbon atoms One of an alkoxy group with a number of 1 to 5, a haloalkyl group with a carbon number of 1 to 5, a nitro group, and a nitrile group; more preferably independently selected from hydrogen, chlorine, fluorine, bromine, iodine, and methoxy One of , methyl, trifluoromethyl, nitro and nitrile;
[0067] R 3 is carboxyl, alkyl, phenyl or cyano; preferably carboxyl, alkyl wit...
Embodiment 1
[0112] Synthesis of compounds A2, b2, c2, d2, e2, f2, g2, h2, i2, j2, k2
[0113] The molar ratio of 1:1.5 o-phenylenediamine or its derivatives and glycolic acid was catalyzed by 4N hydrochloric acid, and reacted at 100°C for 6h. After the reaction, cool to room temperature, adjust the pH to 8 with sodium bicarbonate solution, precipitate a solid, filter it with suction, and dry it by infrared.
[0114]
Embodiment 2
[0116] Synthesis of Compounds A3~A22
[0117] Benzyl bromide with a molar ratio of 1:1 and compound A2 were dissolved in DMF, and a potassium carbonate solid with a molar ratio of benzyl bromide of 1:5 was reacted at room temperature for 8 h. After the reaction, extract with ethyl acetate and water, collect the organic phase, and purify with a silica gel column to obtain a white solid with a yield of 80-93%.
[0118]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com