Novel external drug formula for treating acne and preparation method of formula
A formula and drug technology, applied in the field of medicine, can solve the problems of poor drug stability of acne, complex pharmaceutical composition components, poor treatment effect, etc., and achieve the problem of less impurities, fast dissolution rate, and good acne treatment. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
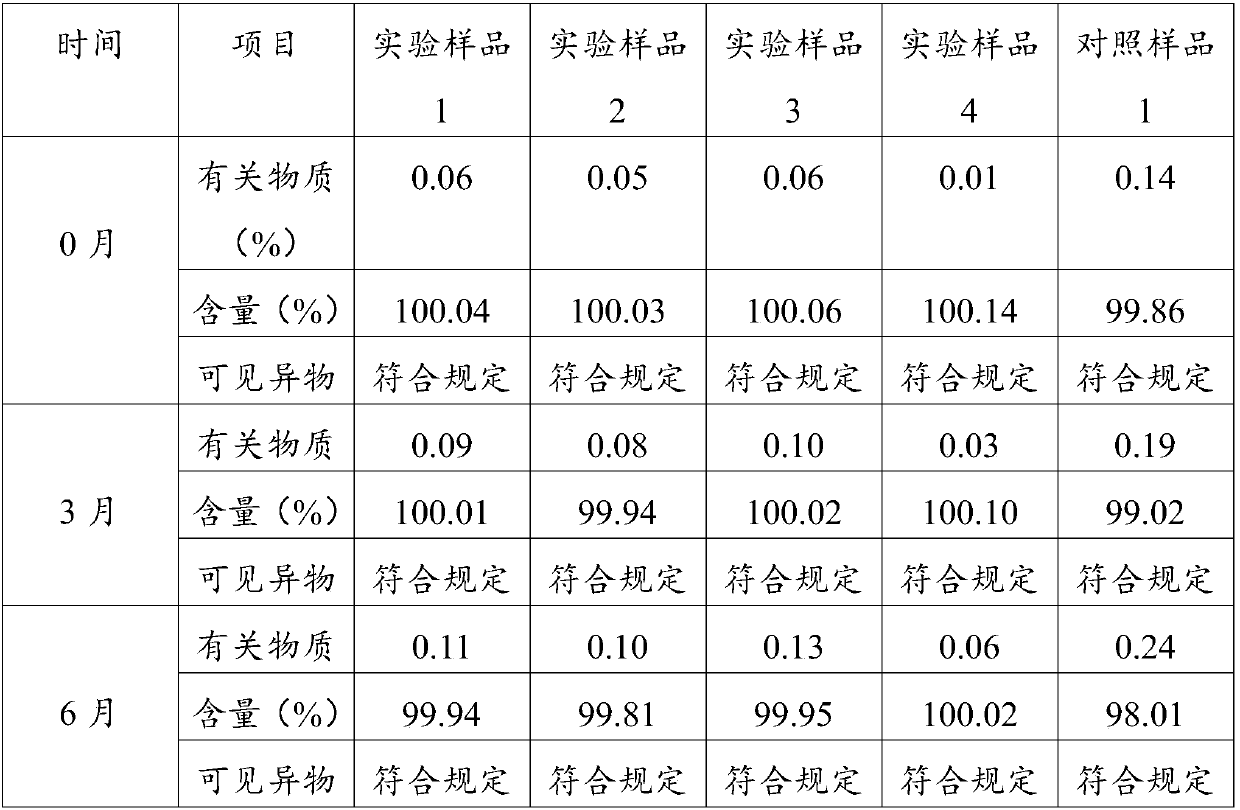
Examples
Embodiment 1
[0037] The preparation of embodiment 1 cimetidine crystal
[0038] (1) Dissolve the cimetidine bulk drug in a mixed solution of methanol and ethanol, wherein the mass volume ratio of the cimetidine bulk drug to methanol and ethanol is 1g: 78ml: 84ml, heat to reflux, and form a a saturated solution;
[0039] (2) Divide the above solution into two cooling treatments, the first cooling to 40°C at a cooling rate of 6°C / min, the second cooling to 4°C at a cooling rate of 3°C / min, and then standing for 24 hours to precipitate white solid, filtered;
[0040] (3) Place the white solid in a vacuum desiccator to dry for 36 hours to obtain the cimetidine crystals.
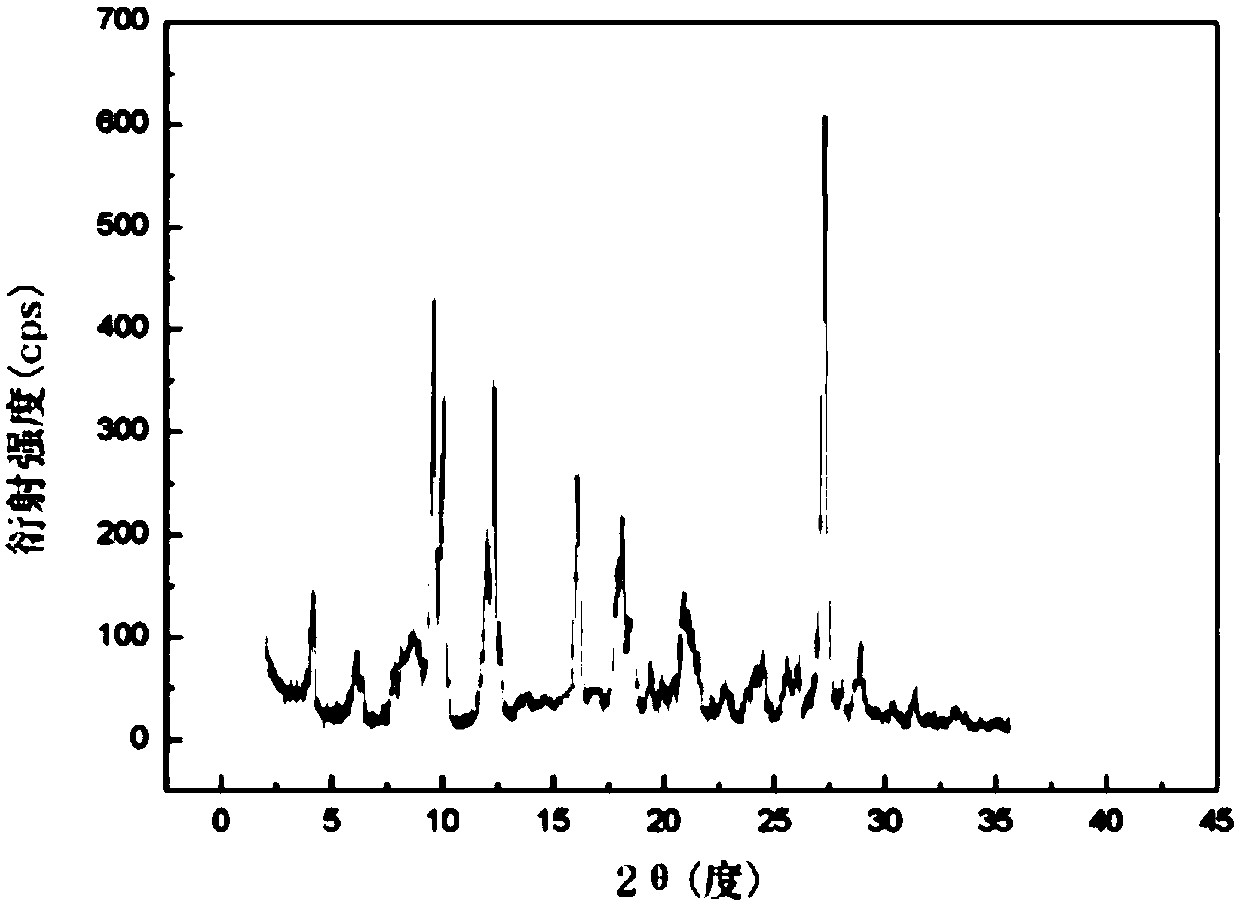
[0041] The X-ray powder diffraction spectrum that cimetidine crystal uses Cu-Kα ray to measure is as figure 1 shown.
Embodiment 2
[0042] The preparation of embodiment 2 cimetidine crystals
[0043] (1) Dissolve the cimetidine bulk drug in a mixed solution of methanol and ethanol, wherein the mass volume ratio of the cimetidine bulk drug to methanol and ethanol is 1g: 80ml: 90ml, heat to reflux, and form a a saturated solution;
[0044] (2) Divide the above solution into two cooling treatments, the first cooling to 43°C at a cooling rate of 7°C / min, the second cooling to 5°C at a cooling rate of 4°C / min, and then standing for 26 hours Precipitate a white solid, filter;
[0045] (3) Place the white solid in a vacuum desiccator to dry for 42 hours to obtain the cimetidine crystals.
[0046]The X-ray powder diffraction spectrum obtained by measuring the obtained cimetidine using Cu-Kα rays is basically consistent with that of Example 1.
Embodiment 3
[0047] The preparation of embodiment 3 cimetidine crystals
[0048] (1) Dissolve the cimetidine bulk drug in a mixed solution of methanol and ethanol, wherein the mass volume ratio of the cimetidine bulk drug to methanol and ethanol is 1g: 83ml: 96ml, heat to reflux, and form a a saturated solution;
[0049] (2) Divide the above solution into two cooling treatments, the first cooling to 45°C at a cooling rate of 8°C / min, the second cooling to 6°C at a cooling rate of 5°C / min, and then standing for 28 hours to precipitate white solid, filtered;
[0050] (3) Place the white solid in a vacuum desiccator to dry for 48 hours to obtain the cimetidine crystals.
[0051] The X-ray powder diffraction spectrum obtained by measuring the obtained cimetidine using Cu-Kα rays is basically consistent with that of Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com