Liquid chromatographic analysis method for simultaneously quantifying glycine and iminodiacetic acid from diethanol amine dehydrogenation product
A technology of iminodiacetic acid and liquid chromatography analysis, applied in the direction of analysis materials, measuring devices, material separation, etc., can solve the problems of toxic derivatization reagents, high derivatization reaction temperature, harsh derivation conditions, etc., and achieve low price and high sensitivity , good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
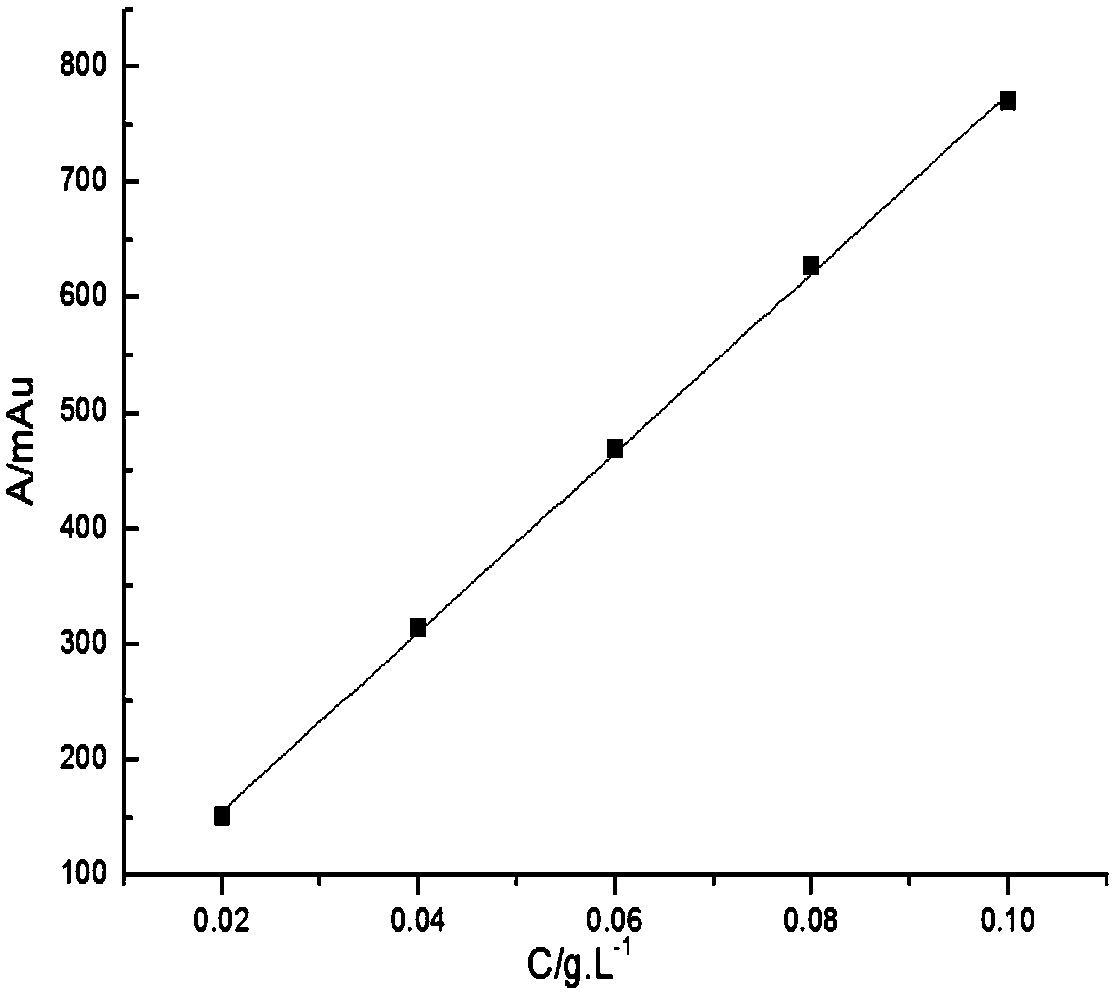
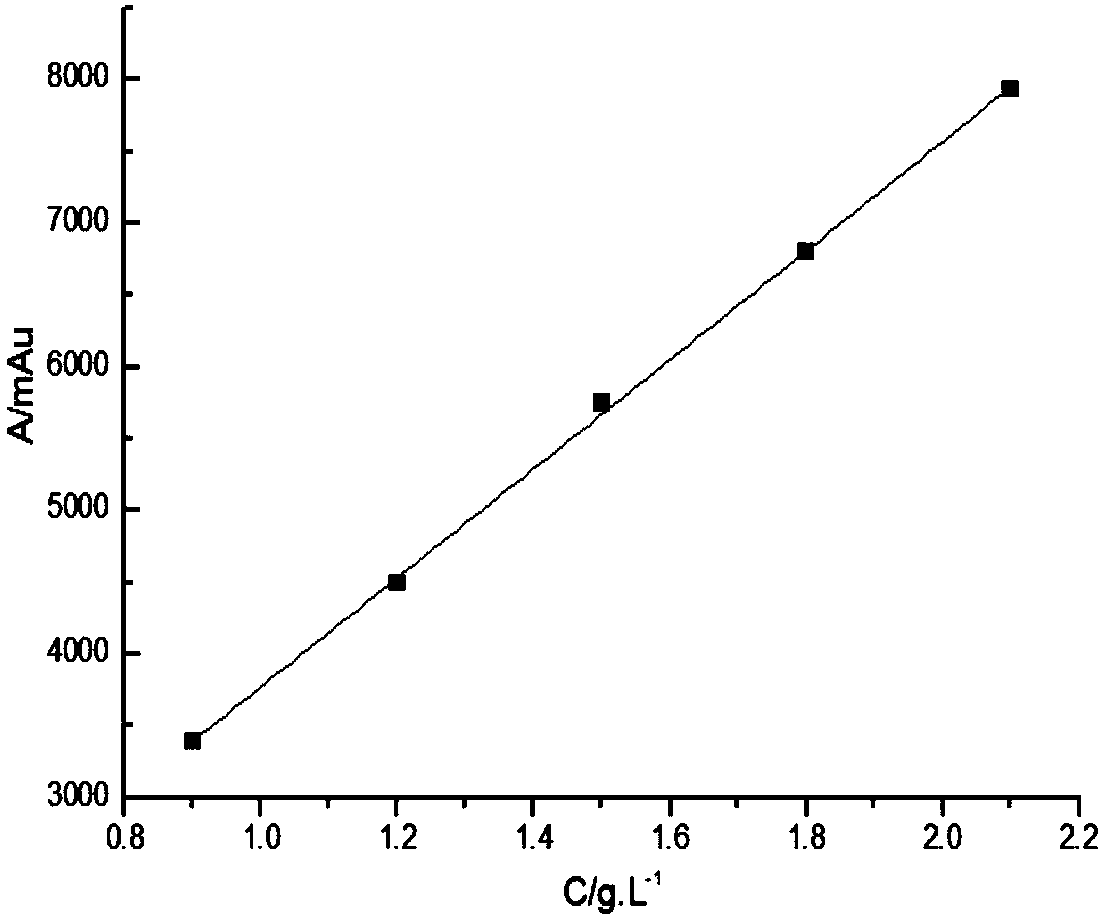
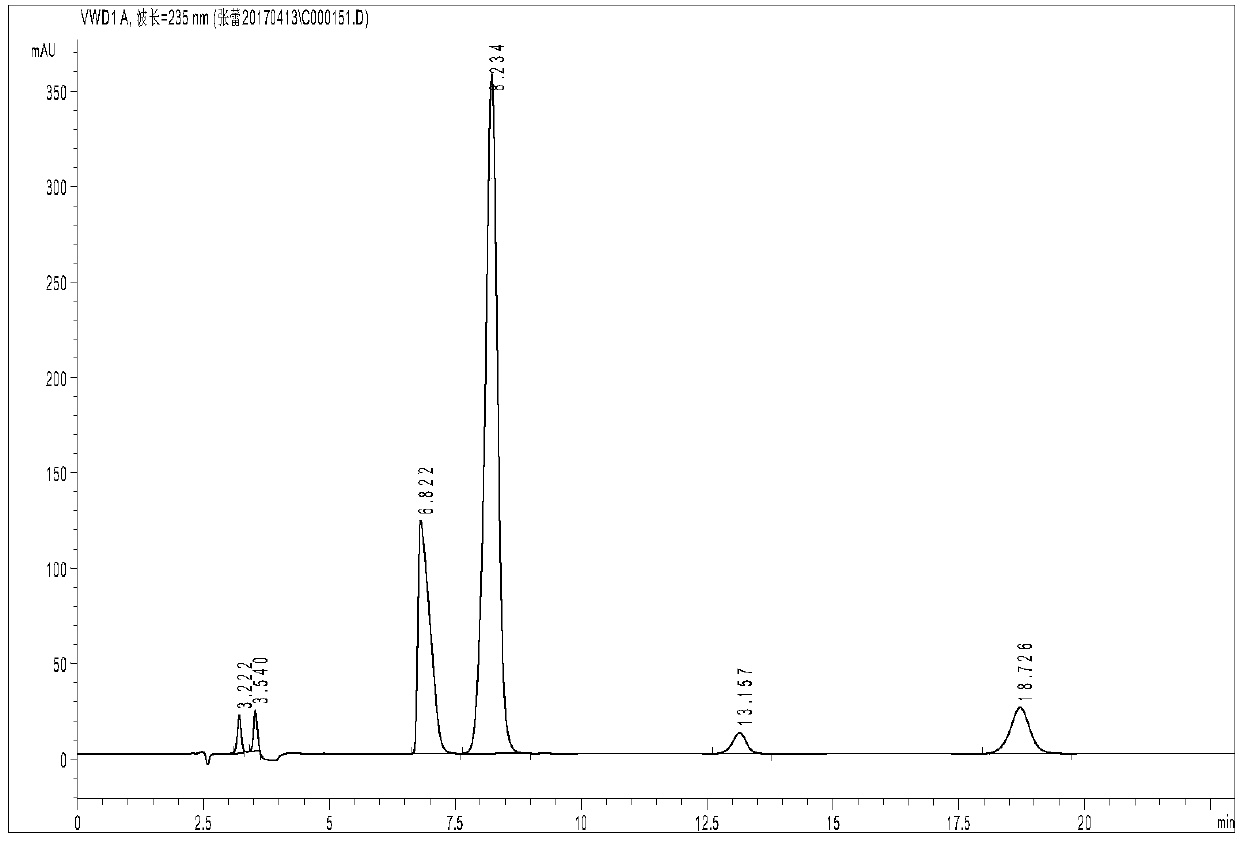
[0037] Glycine and iminodiacetic acid have neither chromogenic groups nor fluorescence, but they belong to primary amines and secondary amines respectively, and are prone to Hisberg reaction; after Hisberg reaction with benzenesulfonyl chloride compounds, they are added to the structure of the compound Introducing chromophore groups to achieve detection in liquid chromatography using ultraviolet absorption; the present invention uses p-toluenesulfonyl chloride as a derivatizing agent, and the reaction principle is shown in the following formula:
[0038]
[0039] Compared with other derivatizing agents, p-toluenesulfonyl chloride has the advantages of low price, rapid reaction and few by-products, but it is inevitable that it will also undergo hydrolysis reaction in water; in order to maximize the inhibition of its hydrolysis reaction, the invention adopts a series of optimizations Experiments have found that the optimal reaction conditions are: borax buffer with a temperature of ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com