2-amino-4,6-dimethoxypyrimidine synthesis method
A technology for the synthesis of dimethoxypyrimidine and its synthesis method, which is applied in the field of synthesis of 2-amino-4,6-dimethoxypyrimidine, can solve the problems of high operating cost, great environmental hazards, complicated drying equipment, etc. The effect of high efficiency, low moisture control and stable product quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
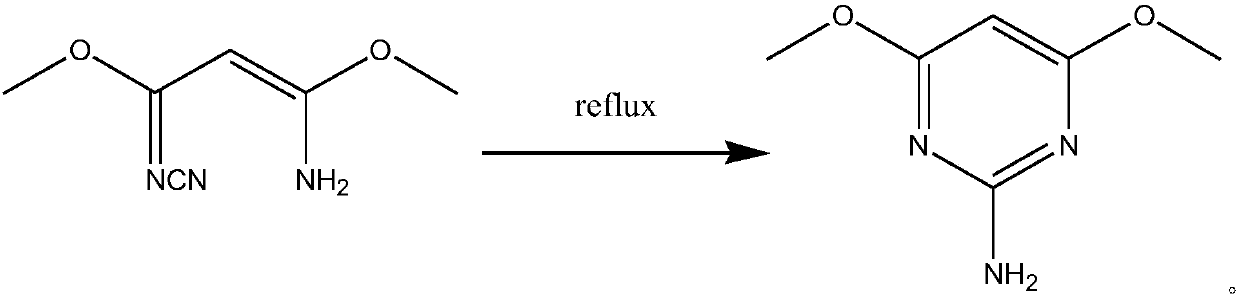
[0023] The synthetic method of 2-amino-4,6-dimethoxypyrimidine, the steps are as follows:
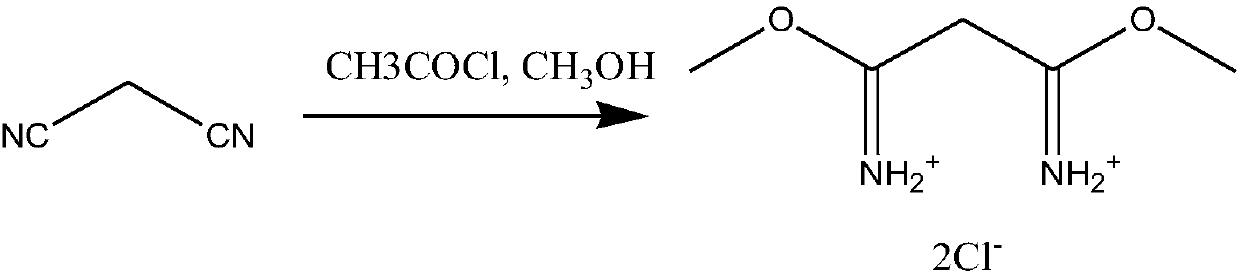
[0024] (1) Synthesis of 1,3‐dimethylmalonamidine dihydrochloride
[0025] Put 66g of malononitrile and 176g of anhydrous methanol into the reaction kettle, slowly drop 235.5g of acetyl chloride into the kettle, control the reaction temperature at 0-5°C, and add the time for 5 hours. After the addition, continue the insulation reaction for 1 Hour, nitrogen protection filtration, obtains 1,3-dimethylmalonamidine dihydrochloride wet product.
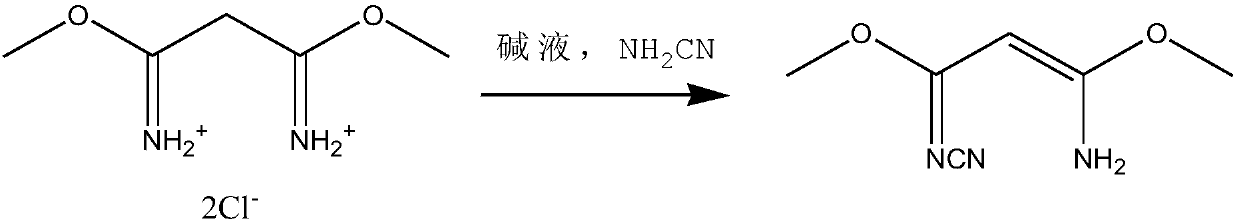
[0026] (2) Synthesis of 3-amino-3-methoxy-N-cyano-2-propionamidine
[0027] Add 1,3‐dimethylmalonimidine dihydrochloride and lye (sodium bicarbonate 20g, sodium hydroxide 30g, water 500g) into the reaction kettle, control the reaction temperature -5-0°C, and slowly add 110g of 50% cyanamide solution, the reaction pH value is 5-6, and the molar ratio of feeding is such that the temperature in the kettle slowly rises to 18°C after the feeding is co...
Embodiment 2
[0032] The synthetic method of 2-amino-4,6-dimethoxypyrimidine, the steps are as follows:
[0033] (1) Synthesis of 1,3‐dimethylmalonamidine dihydrochloride
[0034] Put 66g of malononitrile and 204.8g of anhydrous methanol into the reaction kettle, slowly drop 353.25g of acetyl chloride into the kettle, control the reaction temperature at 10-15°C, and add the time for 8 hours. After the addition, continue the insulation reaction After 2 hours, filter under nitrogen protection to obtain the wet product of 1,3-dimethylmalonamidine dihydrochloride.
[0035] (2) Synthesis of 3-amino-3-methoxy-N-cyano-2-propionamidine
[0036] Add 1,3‐dimethylmalonimidine dihydrochloride and lye (sodium bicarbonate 20g, sodium hydroxide 30g, water 500g) into the reaction kettle, control the reaction temperature at 0-3°C, and slowly add 50 % cyanamide solution 101g, the reaction pH value is 7-8, and the molar ratio of feeding is such that the temperature in the kettle slowly rises to 26° C. after...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com