Method for preparing iron oxide red by using ferrochromium
A technology of red iron oxide and ferric hydroxide, applied in the direction of cells, electrolysis process, electrolysis components, etc., to achieve the effect of simplifying process equipment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
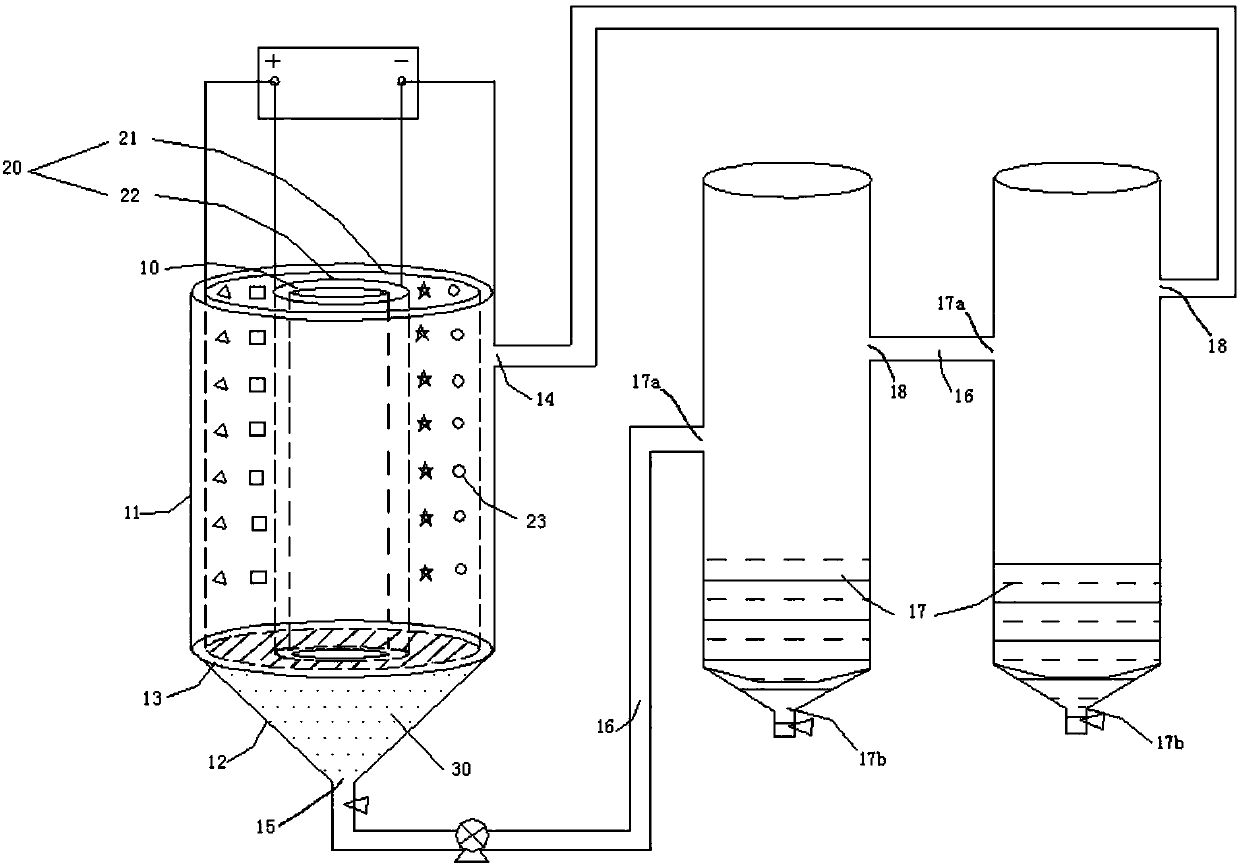
[0091] A stainless steel mesh with an outer diameter of 280 mm and an inner diameter of 80 mm and a stainless steel mesh with an outer diameter of 70 mm are placed in a stainless steel hollow cylinder with an inner diameter of 300 mm. Hollow plastic partitions are used to insulate the titanium mesh and stainless steel. An electrolysis device with double anode and double cathode is formed. Fill the titanium mesh with 100 kg of ferrochrome with a particle size of <100 mm, and fill the electrolytic tank with 44 L of sodium hydroxide solution with a concentration of 260 g / L. The titanium mesh of the electrolytic cell is electrically connected with the positive pole of the power supply, and the stainless steel is electrically connected with the negative pole of the power supply. Turn on the power supply, adjust the output current to 100A, and the cell voltage <2.42V. After 610 hours of continuous electrolytic reaction, the temperature of the electrolyte is 50°C. During this period...
Embodiment 2
[0093]A conductive copper bar is arranged on the long side of a cuboid plastic tank with a length, width, and height of 400mm*500mm*500mm. One side of the copper bar is electrically connected to the positive pole of the power supply, and the other side is electrically connected to the negative pole of the power supply. Place 4 sets of rectangular parallelepiped titanium mesh (400mm*50mm*400mm in length, width, and height) and 5 sets of stainless steel plates (400mm*2mm*400mm in length, width, and height) on the conductive copper bar. The distance between the titanium mesh and the stainless steel plate is 10mm. The titanium mesh is electrically connected to the positive pole of the power supply, and the stainless steel plate is electrically connected to the negative pole of the power supply. Fill each group of titanium mesh with 25kg of ferrochrome with a particle size of <40mm, and fill the plastic tank with 40L of sodium hydroxide solution with a concentration of 110g / L. Turn...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com