Method for synthesizing soybean oligosaccharides through biological catalysis
A technology of soybean oligosaccharide and synthase, applied in the direction of fermentation, can solve the problems of high price, difficulty and low yield of plant extraction method.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Example 1. In vitro multi-enzyme catalyzed conversion of sucrose into galactoside
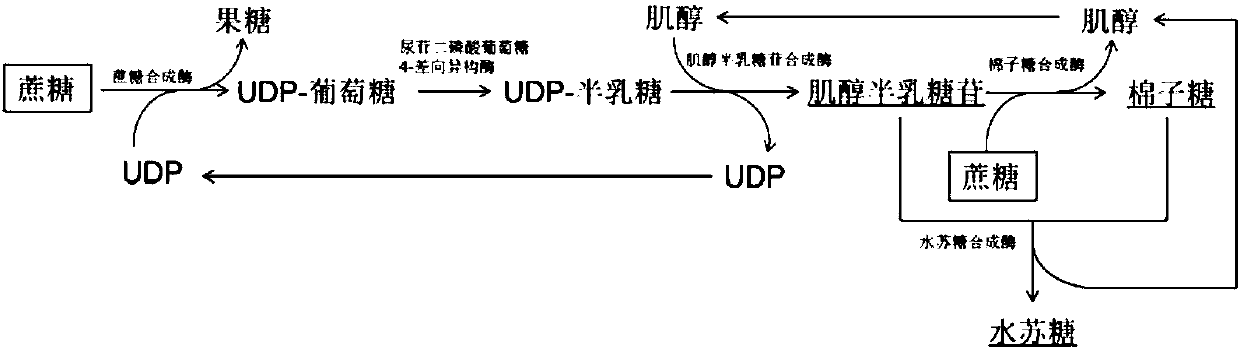
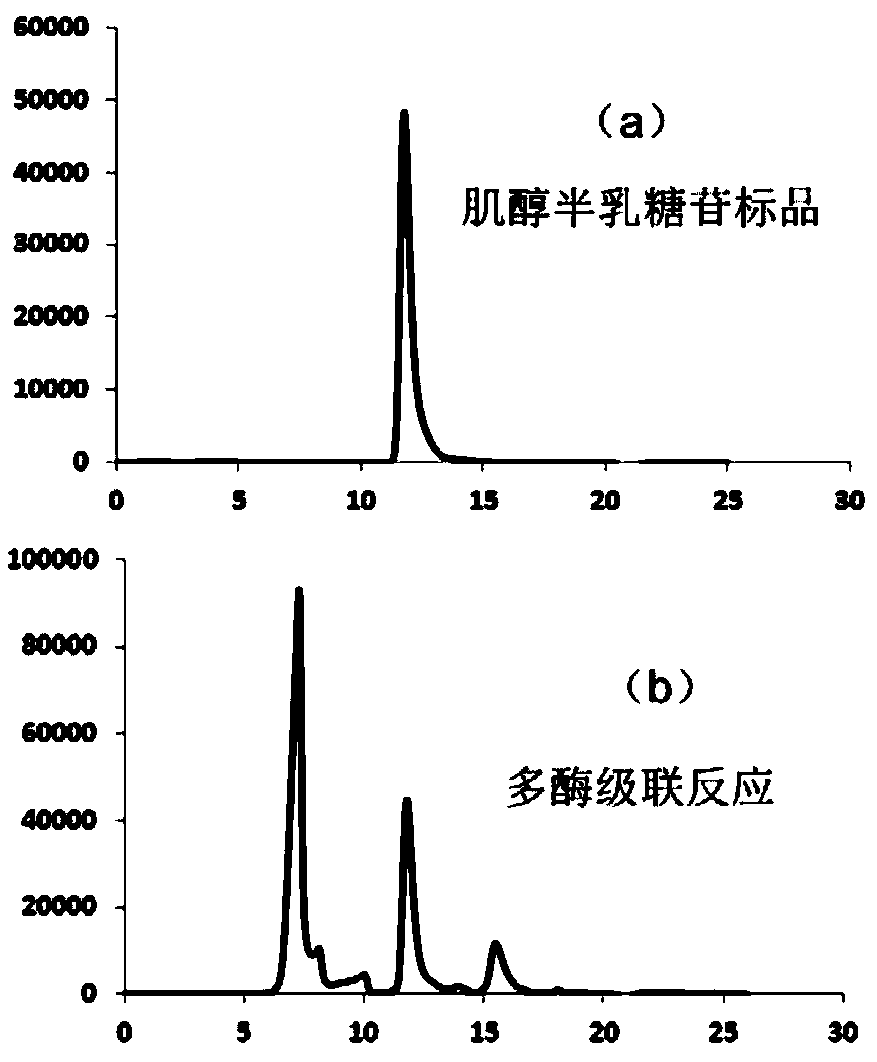
[0052] The conversion of sucrose to galactoside ( figure 1 ). These key enzymes include: (1) sucrose synthase (SS, EC 2.4.1.13), which catalyzes the synthesis of UDP-glucose from sucrose and uridine diphosphate (UDP); (2) uridine diphosphate glucose 4-epimerase (UGE, EC 5.1.3.2), which catalyzes the synthesis of UDP-galactose from UDP-glucose; (3) galactoside synthase (GS, EC 2.4.1.123), which catalyzes the synthesis of inositol hemi-inositol from UDP-galactose and inositol lactose.
[0053] In the present invention, sucrose synthase and galactinol synthase are derived from Arabidopsisthaliana, the gene numbers on KEGG are AT5G20830 and AT1G09350 respectively, and uridine diphosphate glucose 4-epimerase is derived from large intestine The gene number of Escherichia coli on GeneBank is 945354, and these genomic DNAs can be obtained from the official website of ATCC (www.atcc.org). The...
Embodiment 2
[0061] Example 2: In vitro multi-enzyme catalyzed conversion of sucrose into raffinose
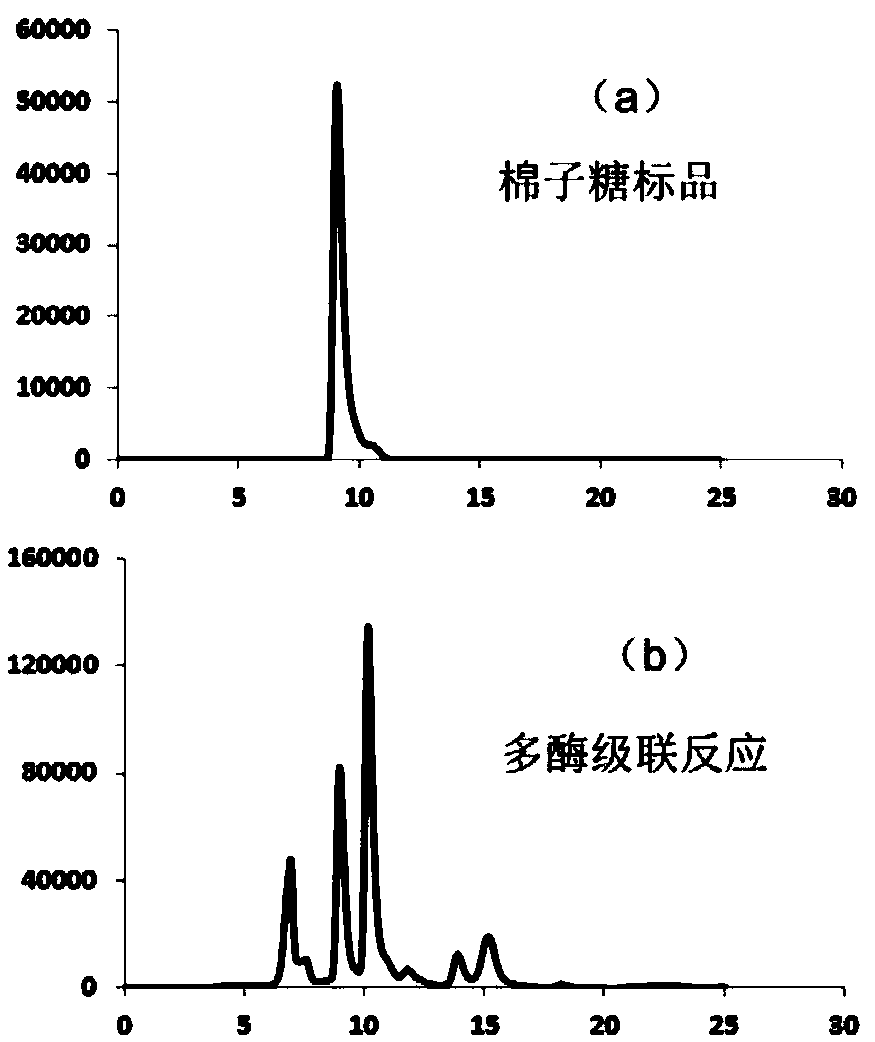
[0062] Establishment of an in vitro multi-enzyme catalytic system to convert sucrose into raffinose ( figure 1 ). These key enzymes include: (1) sucrose synthase (SS, EC 2.4.1.13), which catalyzes the synthesis of UDP-glucose from sucrose and uridine diphosphate (UDP); (2) uridine diphosphate glucose 4-epimerase (UGE, EC 5.1.3.2), which catalyzes the synthesis of UDP-galactose from UDP-glucose; (3) galactoside synthase (GS, EC 2.4.1.123), which catalyzes the synthesis of inositol hemi-inositol from UDP-galactose and inositol Lactose; (4) Raffinose synthase (RS, EC 2.4.1.82), which catalyzes the synthesis of raffinose and inositol from galactositol and sucrose.
[0063] The sources of sucrose synthase, galactinol synthase, and uridine diphosphate glucose 4-epimerase are the same as in Example 1. The raffinose synthase is derived from Arabidopsis thaliana, and the gene is on KEGG The seri...
Embodiment 3
[0068] Example 3, in vitro multi-enzyme catalyzed conversion of sucrose into stachyose
[0069] An in vitro multi-enzyme catalytic system was established to convert sucrose into stachyose ( figure 1 ). These key enzymes include: (1) sucrose synthase (SS, EC 2.4.1.13), which catalyzes the synthesis of UDP-glucose from sucrose and uridine diphosphate (UDP); (2) uridine diphosphate glucose 4-epimerase (UGE, EC 5.1.3.2), which catalyzes the synthesis of UDP-galactose from UDP-glucose; (3) galactoside synthase (GS, EC 2.4.1.123), which catalyzes the synthesis of inositol hemi-inositol from UDP-galactose and inositol Lactose; (4) raffinose synthase (RS, EC 2.4.1.82), which catalyzes the synthesis of raffinose and inositol from galactose inositol and sucrose; (5) stachyose synthase (SS, EC: 2.4.1.67 ), catalyzing the synthesis of stachyose and inositol from galactoside and raffinose.
[0070] The sources of sucrose synthase, galactinol synthase, uridine diphosphate glucose 4-epime...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com