Dynamic polymer elastomer with hybrid crosslinked network and application of dynamic polymer elastomer
A hybrid cross-linking and polymer technology, applied in coatings, adhesives, etc., can solve the problems of lack of dynamics in chemical cross-linking, inability to use elastomers, poor mechanical properties, etc., and achieve excellent energy dissipation performance, good The effect of controllability and excellent self-healing property
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
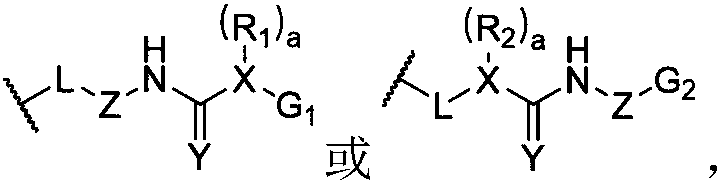
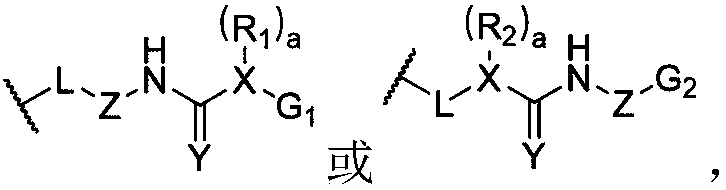
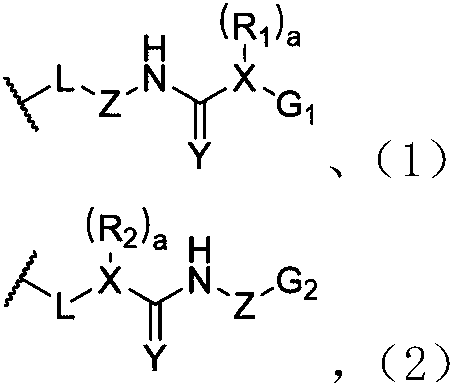
[0127] The preparation method of a kind of dynamic polymer ionic liquid gel of the present invention, comprises the following steps: the raw material of the dynamic polymer that prepares hybrid cross-linked network is added in ionic liquid, makes the dynamic state of prepared hybrid cross-linked network The mass fraction of the polymer is 0.5-70%, and the covalent cross-linking is carried out by the appropriate means, and after the reaction is completed, a dynamic polymer ion liquid gel is prepared. The preparation method of another kind of dynamic polymer ionic liquid gel of the present invention, comprises the following steps: will have the dynamic polymer of hybridization crosslinking network and swelling in the solvent containing ionic liquid, make the prepared hybridization crosslinking The mass fraction of the dynamic polymer in the network is 0.5-70%, and after fully swelling, the solvent is removed to form a dynamic polymer ion liquid gel. The above-mentioned ionic liq...
Embodiment 1
[0172] Mix a certain amount of 5-cyclooctene-1,2-diol and 2-imidazolidinone-4-carboxylic acid, control the molar ratio of the two to be about 1:2, and dicycloethylcarbodiimide and 4-dimethylaminopyridine as a catalyst and dichloromethane as a solvent to obtain the monomer 1a containing a hydrogen bond group.
[0173]
[0174] Mix a certain amount of hydrogen-bonding group-containing monomer 1a with cyclooctene, control the molar ratio of the two to about 1:2, use Grubbs second-generation catalyst as a catalyst, and use dichloromethane as a solvent to obtain a side group Polycyclooctene based polymers containing hydrogen bonding groups.
[0175] Mix 100 parts by mass of the above polymer and 6 parts by mass of dicumyl peroxide in dichloromethane, remove the solvent, place the mixture in a mold and heat it to 150°C for 2 hours, and obtain side group hydrogen bond after cooling Group of polycyclooctene-based dynamic polymer elastomers.
[0176] Mechanical properties: tensile...
Embodiment 2
[0179] React 3-amino-1,2-propanediol and methyl chloroformate in dichloromethane, use anhydrous sodium bicarbonate as a catalyst, and control the molar ratio of amino group to methyl chloroformate to be 10:11 to obtain a side group diols containing carbamate groups.
[0180] Diol containing carbamate groups, adipoyl chloride, butylene terephthalate with hydroxyl groups at both ends, and glycerin are reacted in dichloromethane under the catalysis of triethylamine. Control the molar ratio of diol, adipoyl chloride, and triol to be 50:50:1, and control the molar ratio of diol containing carbamate groups to butylene terephthalate with hydroxyl groups at both ends to be 1 : 1, obtain the dynamic polymer elastomer based on polyester that side group contains carbamate group.
[0181] Mechanical properties: tensile strength 35MPa, elongation at break 1470%.
[0182] This product can be used in fields that require shock absorption, impact resistance, flex resistance and sufficient st...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Tensile strength | aaaaa | aaaaa |
| Tensile strength | aaaaa | aaaaa |
| Tensile strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com