Preparation and application of optical switch fluorescence molecules with hydrogen sulfide recognition function
A technology of fluorescent molecules and optical switches is applied in the field of preparation of fluorescent molecules of optical switches to achieve the effects of good selectivity and strong anti-interference ability of other molecules.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
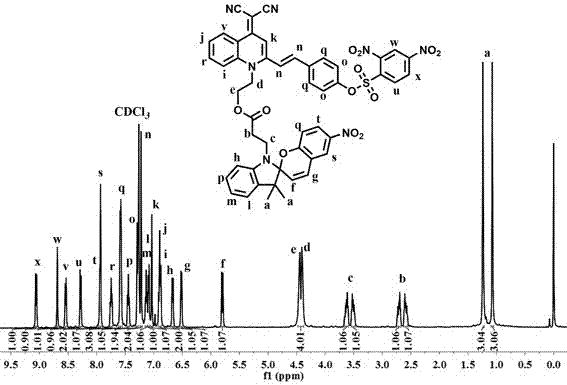
[0042] A preparation of an optical switch molecule with a hydrogen sulfide recognition function, comprising the following steps:
[0043](1) Preparation of the compound of formula II: under the protection of an inert gas, add 2-methylquinoline and 2-bromoethanol into the reaction flask, react at 140°C for 1~3h, cool down to room temperature after the reaction, dissolve with methanol, and Precipitate with ethyl acetate and filter with suction to obtain the compound of formula II; wherein the molar ratio of 2-methylquinoline to 2-bromoethanol is 1:1-1:2.
[0044] (2) Preparation of the compound of formula III: under the protection of inert gas, add the compound of formula II and malononitrile into the reaction flask, use ethanol as the solvent, add sodium ethylate dropwise under ice bath, react for 1~3h, and react at room temperature for 4~ After 18 hours, after the reaction was completed, suction filtered to obtain a yellow filter cake, which was washed with ethanol to obtain t...
Embodiment 2
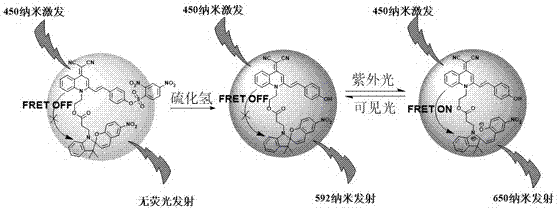
[0049] Embodiment 2: detection experiment of hydrogen sulfide.
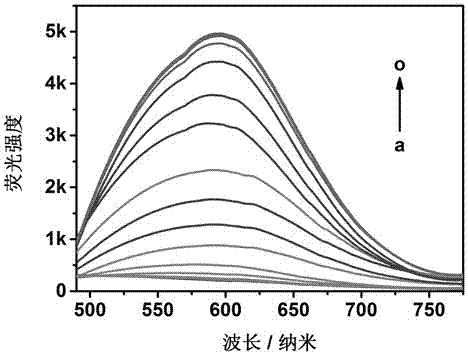
[0050] Take 15 5 mL sample vials, add 3 mL of the solution prepared by the molecule of formula I obtained in Example 1 (the concentration of the molecule is 10 μM, V tetrahydrofuran: V water = 90: 10), and then respectively add the concentration of [ HS - ] = 0(a), 1.0×10 -3 mol / L(b), 2.0×10 -3 mol / L(c), 5.0×10 -3 mol / L(d), 1×10 -2 mol / L(e), 2.0×10 - 2 mol / L(f), 3.0×10 -2 mol / L (g), 4×10 -2 mol / L(h), 5×10 -2 mol / L(i), 6×10 -2 mol / L(j), 7×10 -2 mol / L (k), 8×10 -2 mol / L(l), 9×10 -2 mol / L(m), 1.0×10 -1 mol / L l(n), 1.1×10 -1 mol / L (o) 3 µL hydrogen sulfide solution was added to 15 sample bottles, stirred at room temperature for 90 minutes, and the fluorescence intensity of these samples was measured with 450 nm as the excitation wavelength, and the fluorescence emission spectrum changes of the 15 samples were obtained. ,See image 3 . The measurement results show that the fluorescence in...
Embodiment 3
[0051] Example 3: Comparative detection experiments of other ions and molecules.
[0052] Take 19 5 mL sample vials, add 3 mL of the solution prepared by the molecule of formula I obtained in Example 1 (the concentration of the molecule is 10 μM, V tetrahydrofuran: V water = 90: 10), and then respectively add the concentration of 5.0 ×10 -1 mol / L Cl - (1), F - (2), CO 3 2- (3), H 2 PO 4 - (4), HCO 3 - (5), HSO 4 - (6), NO 3 - (7), OAc - (8), PO 4 3- (9), SO 4 2- (10), ClO - (11), H 2 o 2 (12), NO 2 - (13), SO 3 2- (14), GSH(15), Cys(16), Hcy(17), and HS - (18) Take 3 µL each and add to the other first 18 sample vials, and sample No. 19 is the blank sample. Then measure the fluorescence emission intensity at 450 nm wavelength excitation and 592 nm wavelength emission of 19 samples respectively, the results are shown in Figure 5 . The measurement results show that, except hydrogen sulfide, other above-mentioned various ions and molecules have no obvi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com