Litopenaeus vannamei haemocyanin antibacterial peptide and applications thereof
A technology of hemocyanin and antimicrobial peptides, which can be applied in the direction of antibacterial drugs, peptide/protein components, peptides, etc., can solve the problems of low efficiency of shrimp farming industry, insufficient development momentum, and unoptimistic prospects for sustainable development.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
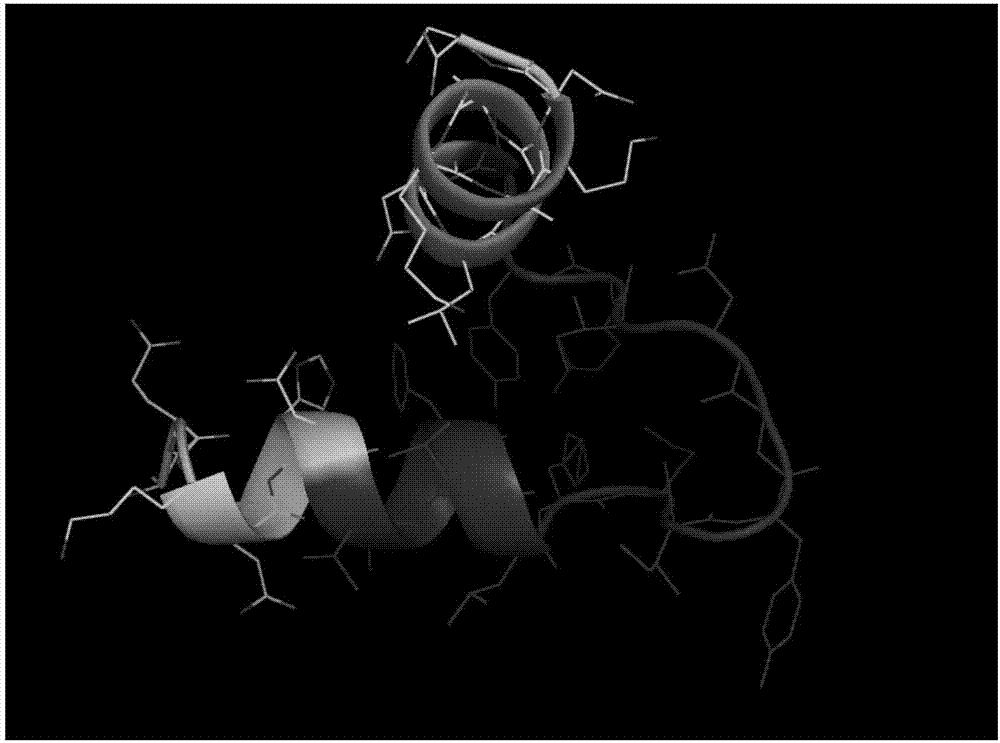
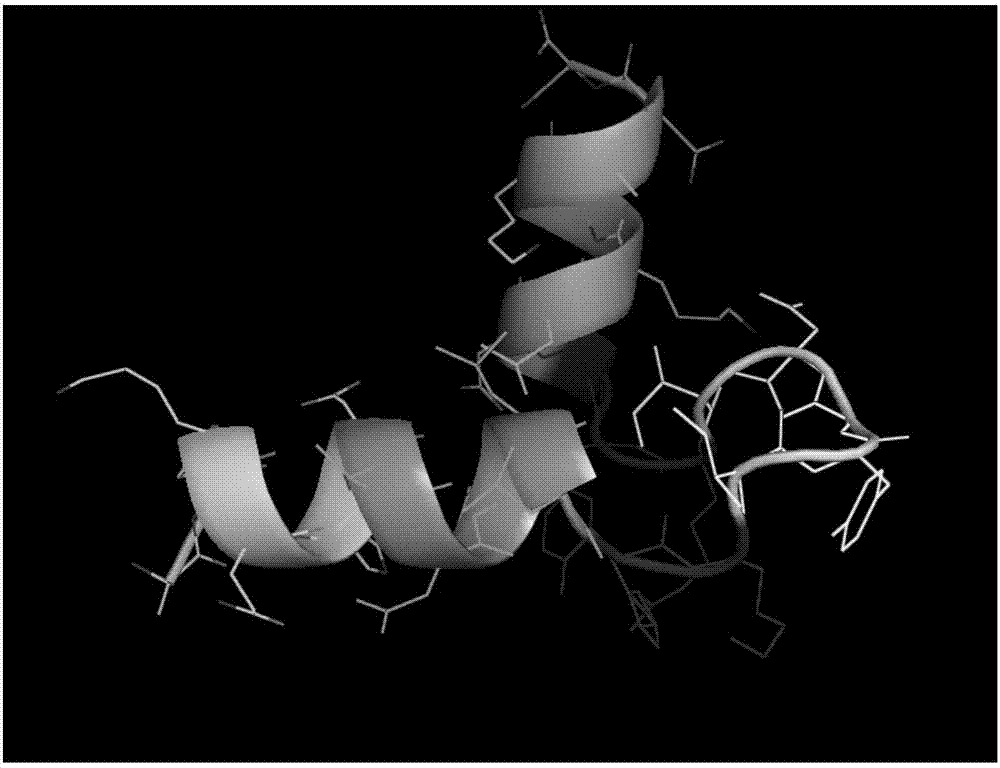
[0020] Example 1 Prediction of Litopenaeus vannamei Hemocyanin Antimicrobial Peptide
[0021] Firstly, three online prediction software, AntiBP Server, CAMP, and APD2, were used to predict the large and small subunit antimicrobial peptides of Litopenaeus vannamei hemocyanin, as shown in Table 1 and Table 2. A total of 34 antibacterial peptides with possible antibacterial activity were predicted Small molecular fragments, among which 11 polypeptide sequences (indicated in bold) may form an α-helical structure and can interact with pathogenic bacteria membranes to exert antibacterial effects. The predicted molecular weight of these polypeptides ranges from 1.5 to 1.9 kDa. In addition, the prediction results showed that there were active fragments in the α-helical domain at the N-terminus of hemocyanin, the Ig-like domain at the C-terminus, and the conserved copper ion-binding domain in the middle, of which the copper-ion-binding domain and the Ig-like domain accounted for the mai...
Embodiment 2
[0030] Example 2 Analysis of Litopenaeus vannamei Hemocyanin Antimicrobial Peptide Secondary Structure Content by Circular Dichroism
[0031] The 11 peptides described in Implementation 1 (the bolded peptides in Table 1 and Table 2) that may form an α-helical structure and can interact with pathogenic bacteria membranes to exert antibacterial effects were scanned by circular dichroism chromatography, and analyzed its structural content.
[0032] 2.1 Experimental equipment
[0033] 2.1.1 Experimental materials
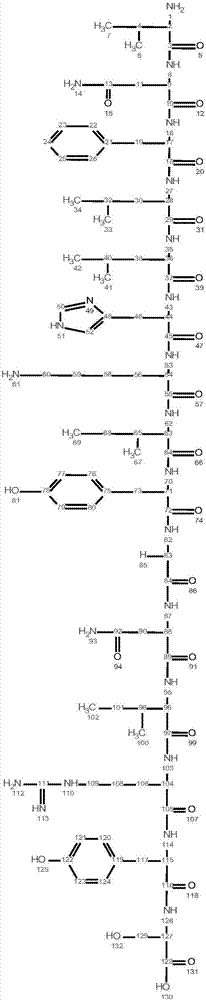
[0034] The polypeptide used in the experiment was synthesized by Beijing Zhongke Yaguang Biotechnology Co., Ltd. The N-terminal acetylation and C-terminal amidation of the polypeptide were modified, and the purity was >95%. A total of 11 antimicrobial peptides, numbered S7, S8, S9, S10, B1, B2, B3, B9, B11, B13, B14.
[0035] 2.1.2 Experimental Instruments
[0036] Ultraviolet spectrophotometer (UV5200) manufacturer: Shanghai Yuanxi Instrument Co., Ltd.
[0037] Ci...
Embodiment 3
[0075] Embodiment 3 adopts plate count method to verify the bacteriostatic activity of antimicrobial peptide B2
[0076] 3.1 Experimental materials
[0077] 3.1.1 Experimental materials
[0078] The polypeptide used in the experiment was synthesized by Beijing Zhongke Yaguang Biotechnology Co., Ltd. The N-terminal acetylation and C-terminal amidation of the polypeptide were modified, and the purity was >95%.
[0079] The pathogenic bacteria in the experimental aquatic products were Vibrio, Alginolyticus, Escherichia coli, Aeromonas hydrophila, Vibrio parahaemolyticus, Streptococcus B and Staphylococcus aureus, all of which were preserved in our laboratory. strains.
[0080] 3.1.2 Main reagents
[0081] Bovine tryptone, beef extract, agar powder, sodium chloride, and sodium hydroxide were purchased from Guangzhou Weijia Technology Co., Ltd.
[0082] 3.1.3 Solution preparation
[0083] 3.1.3.1 Artificially synthesized peptide solution
[0084] 1.0 mg / mL peptide solution: t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
| Absorbance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com