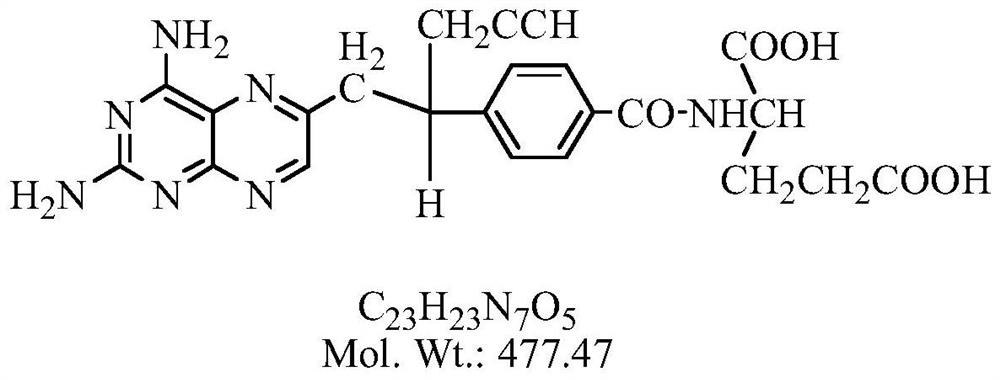
A kind of preparation method of pralatrexate intermediate
A technology of pralatrexate and intermediates, applied in the field of medicine, can solve the problems of low yield, long reaction time, low yield and purity, etc., to improve yield and purity, simplify post-treatment process, and improve operability sexual effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Under the protection of argon, add 323g PLQS-1 and 2.26L THF into a 5L three-necked flask, and when the temperature is lowered and stirred to -10°C, pour 224ml of 3-bromopropyne into the reaction flask, keep stirring for 0.5h, and start to drop 1.8L THF solution of 1mol / L LiHMDS, control the dropping rate, and keep the reaction temperature at -10±3°C (about 2 to 3 hours to complete the dropping). After the dropwise addition, continue to stir for 0.5 h, and detect by HPLC. After the reaction is complete, pour the reaction liquid into 1.25 L of acetic acid aqueous solution, stir for 10 min, and then let stand to separate the liquids. Add about 1.3L of isopropanol, stir to precipitate a solid, then put it in a cold bath at 0-5°C, stir for 3-4h, filter, and wash with a mixed solvent of about 300ml of isopropanol: n-heptane = 1:6 filter cake. The filter cake was oven-dried at 50°C to finally obtain 332g of light yellow solid with a yield of 87.0%. The HPLC detection of the ...
Embodiment 2
[0028] Under the protection of argon, add 323g PLQS-1 and 2.26L THF into a 5L three-necked flask, cool down and stir to -20°C, pour 224ml 3-bromopropyne into the reaction flask, keep stirring for 0.5h, and start to drop 1.8L 1mol / L. THF solution of LiHMDS, control the rate of addition, and keep the reaction temperature at -20±3°C (about 2 to 3 hours to complete the drop). After the dropwise addition, continue to stir for 0.5 h, and detect by HPLC. After the reaction is complete, pour the reaction liquid into 1.25 L of acetic acid aqueous solution, stir for 10 min, and then let stand to separate the liquids. Add about 1.3L of isopropanol, stir to precipitate a solid, then put it in a cold bath at 0-5°C, stir for 3-4h, filter, and wash with a mixed solvent of about 300ml of isopropanol: n-heptane = 1:6 filter cake. Filter cake is oven-dried at 50 ℃, finally obtains 320g light yellow solid, yield is 83.8%, the HPLC detection of intermediate α-propargyl-(4-formic acid methyl este...
Embodiment 3
[0030] Under the protection of argon, add 323g PLQS-1 and 2.26L ethyl acetate into a 5L three-necked flask, cool down and stir to -10°C, pour 224ml 3-bromopropyne into the reaction flask, keep stirring for 0.5h, start Add 1.8L of 1mol / L.LiHMDS THF solution dropwise, control the dropping rate, and keep the temperature of the reaction bottle at -10±3°C (about 2 to 3 hours to complete the dropping). After the dropwise addition, continue to stir for 0.5 h, and detect by HPLC. After the reaction is complete, pour the reaction liquid into 1.25 L of acetic acid aqueous solution, stir for 10 min, and then let stand to separate the liquids. Add about 1.3L of isopropanol, stir to precipitate a solid, then put it in a cold bath at 0-5°C, stir for 3-4h, filter, and wash with a mixed solvent of about 300ml of isopropanol: n-heptane = 1:6 filter cake. Filter cake is oven-dried at 50 ℃, finally obtains 330g light yellow solid, yield is 86.4%, the HPLC detection of intermediate α-propargyl-(...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com