A lithium-oxygen battery
A technology of lithium-oxygen battery and lithium negative electrode, applied in battery pack parts, battery box/jacket, large-sized battery/battery pack, etc., can solve the problems of accelerated battery failure, unstable lithium negative electrode, poor cycle stability, etc. , to protect the lithium metal anode, avoid additive depletion, and achieve high cycle reversibility.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
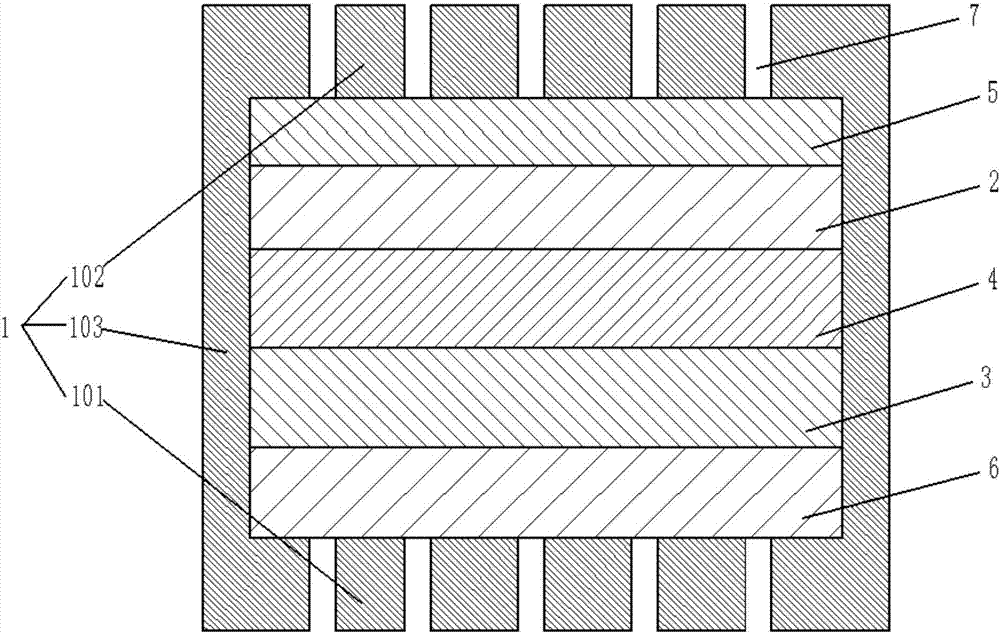
[0026] see figure 1 , a lithium-oxygen battery according to the present invention, comprising a casing 1, a porous lithium negative electrode 2 disposed in the casing 1, a porous positive electrode 3, and a porous lithium negative electrode 2 disposed between the porous lithium negative electrode 3 and the porous positive electrode 3. The diaphragm 4 has an electrolyte solution. In this embodiment, the electrolyte solution is preferably added dropwise. The electrolyte solution can be selected from ether solvents including tetraethylene glycol dimethyl ether and triethylene glycol dimethyl ether. The lithium salt can be selected from lithium perchlorate, lithium bistrifluoromethanesulfonyl imide, lithium trifluoromethanesulfonate, lithium nitrate and the like. The molar ratio of solvent to lithium salt is between 1-8:1. In this embodiment, an electrolyte solution with a molar ratio of tetraethylene glycol dimethyl ether to lithium trifluoromethanesulfonate of 4:1 is preferred....
Embodiment 2
[0031] All the other structures of the lithium-oxygen battery are the same as in Example 1, except that:
[0032] The porous positive electrode in Example 1 was replaced with a porous metal lithium sheet, and the porous positive electrode current collector was replaced by a foamed copper current collector from an aluminum mesh current collector, thereby converting the lithium-oxygen battery into a lithium-lithium battery.
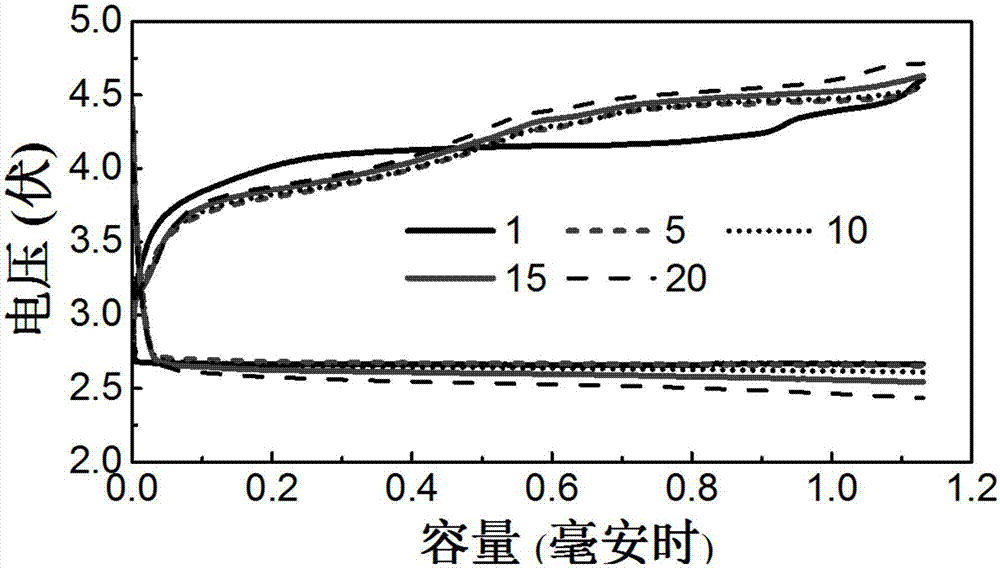
[0033] Put the above-mentioned modified lithium-lithium battery in a bottle filled with pure oxygen for charge and discharge test. In addition, a reference battery without opening holes for oxygen to enter the battery at the negative and positive electrodes participated in the test together, and the following results were obtained: image 3 the results described. At a current density of 0.2mA / cm 2 In this case, the time for lithium metal deposition and dissolution was controlled within 5 hours. As the cycle progressed, the overpotential of the battery wit...
Embodiment 3
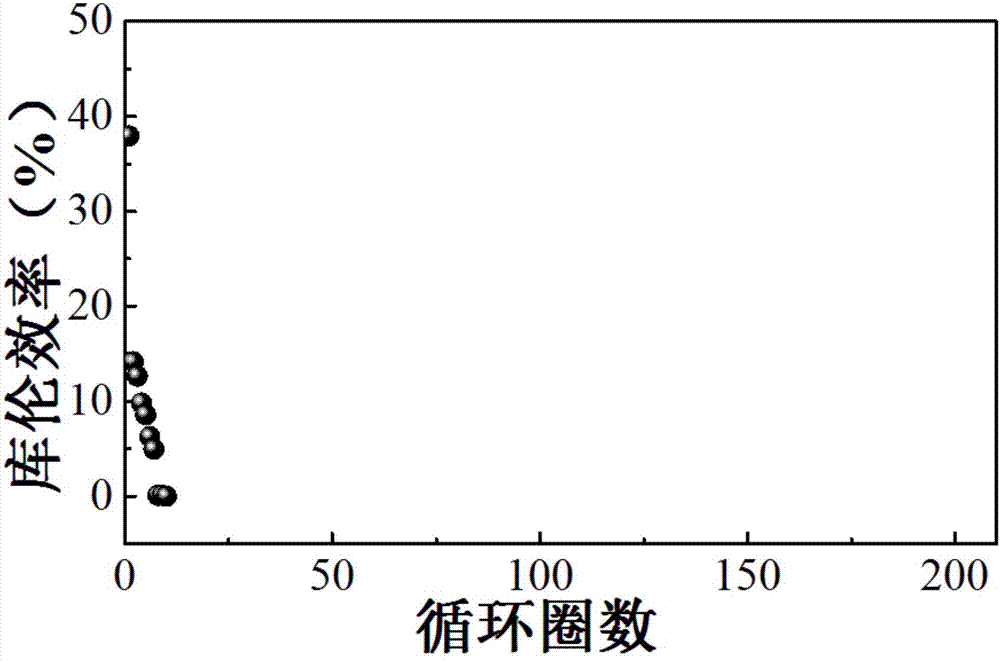
[0035] The rest of the structure of the lithium-oxygen battery is the same as that of Example 2, except that the porous metal lithium sheet and the foamed copper current collector on one side of the lithium-oxygen battery described in Example 2 are replaced with a copper mesh so that the lithium-lithium battery is converted into Lithium-copper battery. In this embodiment, a certain capacity of lithium metal is deposited on the copper grid, and then the deposited lithium is dissolved, and the coulombic efficiency of lithium in the cycle process can be known by calculating the ratio of the dissolved lithium to the deposited lithium. In the experiment, the diameter of the electrode sheet we used was 12mm, and the amount of deposited metal lithium was 1mAh / cm 2 , The charging cut-off potential is 1.0V. The lithium-oxygen battery in this embodiment is placed in a bottle full of pure oxygen for testing, and the following results are obtained: Figure 4 The cycle graph shown. It c...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Aperture | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com