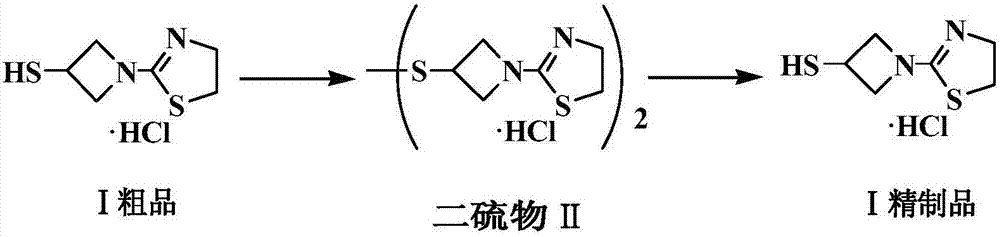
Refining method of side chain of tebipenem pivoxil
A technology of tipipenem ester and a purification method, applied in the field of organic drug synthesis, can solve the problems of low product purity and yield, difficult crystallization and the like, and achieve the effects of simple operation, reduced production cost and mild conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Add 29.8g tipipenem ester side chain crude product and 0.1g potassium iodide into 60ml water, add saturated potassium iodate solution dropwise at 30°C, after TLC (thin layer chromatography) detects that the reaction is over, add 60ml dichloromethane, stir After 10 minutes, separate the liquid, discard the organic layer, adjust the pH value of the aqueous layer to 9-10 with 30% sodium hydroxide solution, filter, and dry to obtain 16.5 g of white solid, with a yield of 95.3%.
[0029] In a 250ml three-necked flask, add 13.9g (40mmol) of the white solid obtained in the previous step, 11.0g (42mmol) of triphenylphosphine, 8.5g of 36% hydrochloric acid (HCl84mmol) and 70ml of ethanol, stir and heat up to 30°C, heat preservation reaction, TLC The reaction was monitored until conversion of starting material was complete. The reaction solution was heated up to 50°C and concentrated to dryness under reduced pressure. After it was hot, 20ml of ethanol was added to dissolve it. Af...
Embodiment 2
[0031] Add 10.4g of tipipenem ester side chain crude product and 0.05g of potassium iodide into 30ml of water, add saturated potassium iodate solution dropwise at 30°C, after the reaction is detected by TLC, add 30ml of n-butanol, stir for 10min, separate the liquid, discard The organic layer and the aqueous layer were adjusted to pH 8-9 with sodium carbonate solution, filtered, and dried to obtain 7.8 g of white solid, with a yield of 90.0%.
[0032] In a 100ml three-necked flask, add 7.6g (22mmol) of the white solid obtained in the previous step, 6.0g (23mmol) of triphenylphosphine, 4.7g of 36% hydrochloric acid (HCl 46.2mmol) and 45ml of ethanol, stir and heat up to 30°C, and keep the temperature for reaction , TLC monitored the reaction until the conversion of the starting material was complete. The reaction solution was heated to 60°C and concentrated to dryness under reduced pressure. After it was hot, 13ml of n-butanol was added to dissolve it. After the temperature dro...
Embodiment 3
[0034] Add 44.3g of tipipenem ester side chain crude product and 0.1g of sodium iodide into 90ml of water, add saturated potassium iodate solution dropwise at 30°C, after the reaction is detected by TLC, add 90ml of ethyl acetate, stir for 10min, and separate the liquids , the organic layer was discarded, and the pH value of the aqueous layer was adjusted to 11-12 with sodium bicarbonate solution, filtered, and dried to obtain 25.1 g of a white solid, with a yield of 96.5%.
[0035]In the there-necked flask of 100ml, add 22.8g (66mmol) last step gained white solid, 18.0g (69mmol) triphenylphosphine, 2.4g (132mmol) water, 66ml ethanol and 2M HCl ethanolic solution 69ml (HCl 138mmol), stir The temperature was raised to 30° C., and the reaction was carried out with heat preservation, and the reaction was monitored by TLC until the conversion of the raw materials was complete. The reaction solution was heated up to 55°C and concentrated to dryness under reduced pressure. After it ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com