Method for synthesizing 2-bromo-1,1,2,2-tetrafluoroethyl substituted aryl building block
The technology of a tetrafluoroethyl group and a synthesis method is applied in the field of synthesis of aryl building blocks, and achieves the effects of short reaction steps, simple and easy-to-obtain raw materials and reagents, and green sources.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
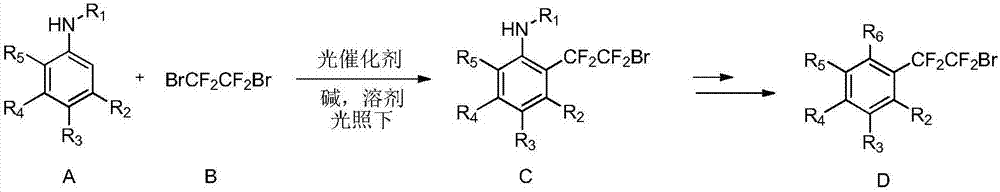
[0033] The invention provides a method for synthesizing dibromotetrafluoroethane-substituted aniline and its derivatives. Preferably, the method includes the step of: in an organic solvent, at a certain temperature (such as 0°C-80°C; preferably 10°C-50°C), under the irradiation of blue light or green light in visible light, to contain iridium , the complex of ruthenium is a photocatalyst, and the compound of formula A (i.e. aniline or its derivatives) is reacted with the compound of formula B) for a period of time (such as 1 to 40 hours) to form the compound of formula C (dibromotetrafluoroethane substituted Aniline and derivatives thereof), and D compounds can be obtained through some classic organic reactions;
[0034]
[0035] In various forms, R 1 , R 2 , R 3 , R 4 , R 5 , R 6 Defined as above.
[0036] More preferably, the compound of formula A is a compound selected from the group consisting of:
[0037]
[0038] Compounds of formula A and formula B of the ...
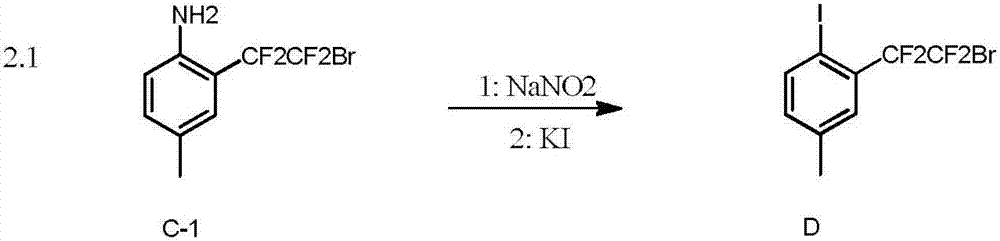
Embodiment 1
[0050]
[0051] To a 25mL reaction tube, add 1.3mg (0.5mol%) Ir(PPy), Na 2 CO 3 (0.4mmol), compound A-1 (102mmol, 3 equivalents), after argon replacement three times, add 2mL of acetonitrile (MeCN), inject 50μL (0.40mmol) of compound B-1, and stir for 24 hours under blue light irradiation to obtain compound C-1, 78% yield. 1 HNMR (400MHz, CDCl 3 )δ7.13(1H), 7.10(d, J=8.4Hz, 1H), 6.62(d, J=8.4Hz, 1H), 3.81(br, 2H), 2.26(s, 3H). 19 FNMR (376MHz, CDCl 3 ) δ - 64.5 (t, J = 5.6Hz, 2F), -105.4 (t, J = 5.6Hz, 2F).
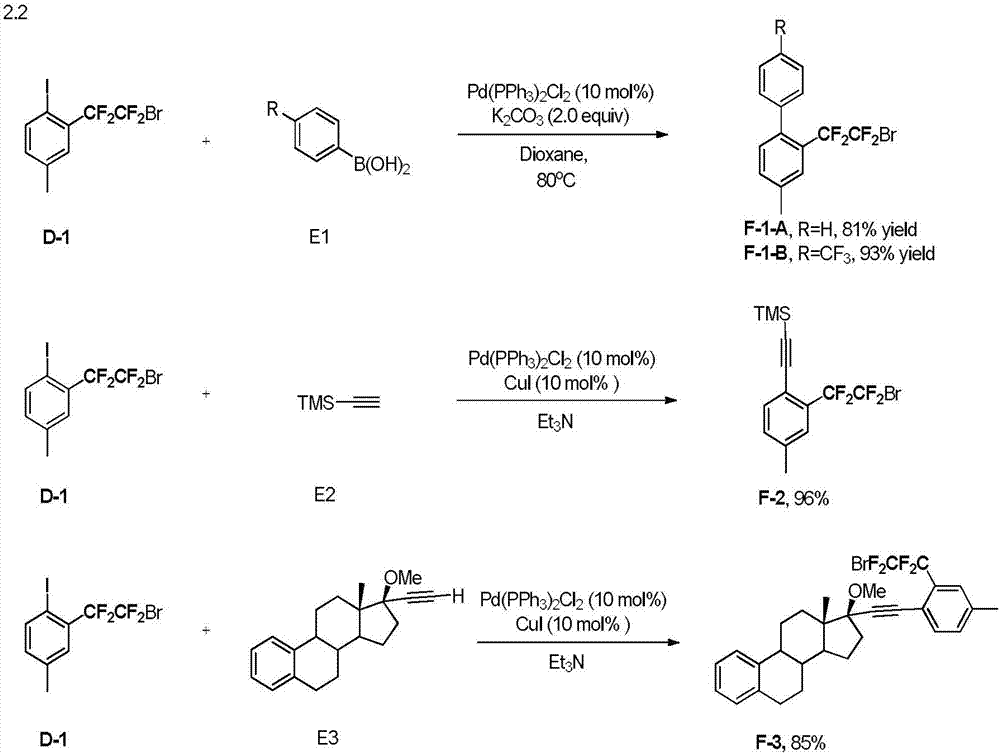
Embodiment 2
[0053]
[0054] Into a 25mL reaction tube, add 1.3mg (0.5mol%) Ir(PPy) 3 , K 3 PO 4 (0.4mmol), compound A-1 (1.2mmol, 3 equivalents), nitrogen replacement three times, adding 2mL of acetonitrile (MeCN), injecting 50μL (0.40mmol) of compound B-1, and stirring for 24 hours under blue light irradiation, to obtain compound C-1, 65% yield. 1 HNMR (400MHz, CDCl 3 )δ7.13(1H), 7.10(d, J=8.4Hz, 1H), 6.62(d, J=8.4Hz, 1H), 3.81(br, 2H), 2.26(s, 3H). 19 FNMR (376MHz, CDCl 3 ) δ - 64.5 (t, J = 5.6Hz, 2F), -105.4 (t, J = 5.6Hz, 2F).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com