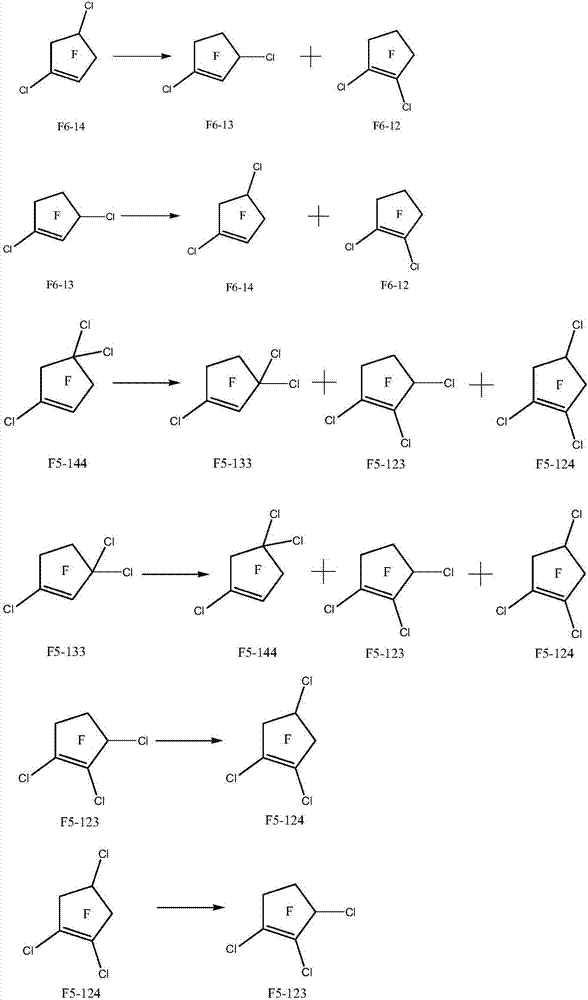
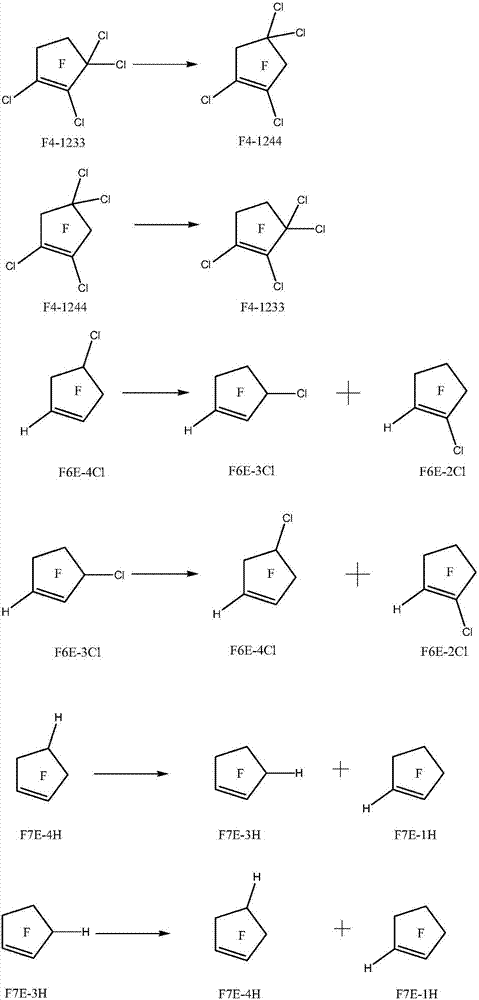
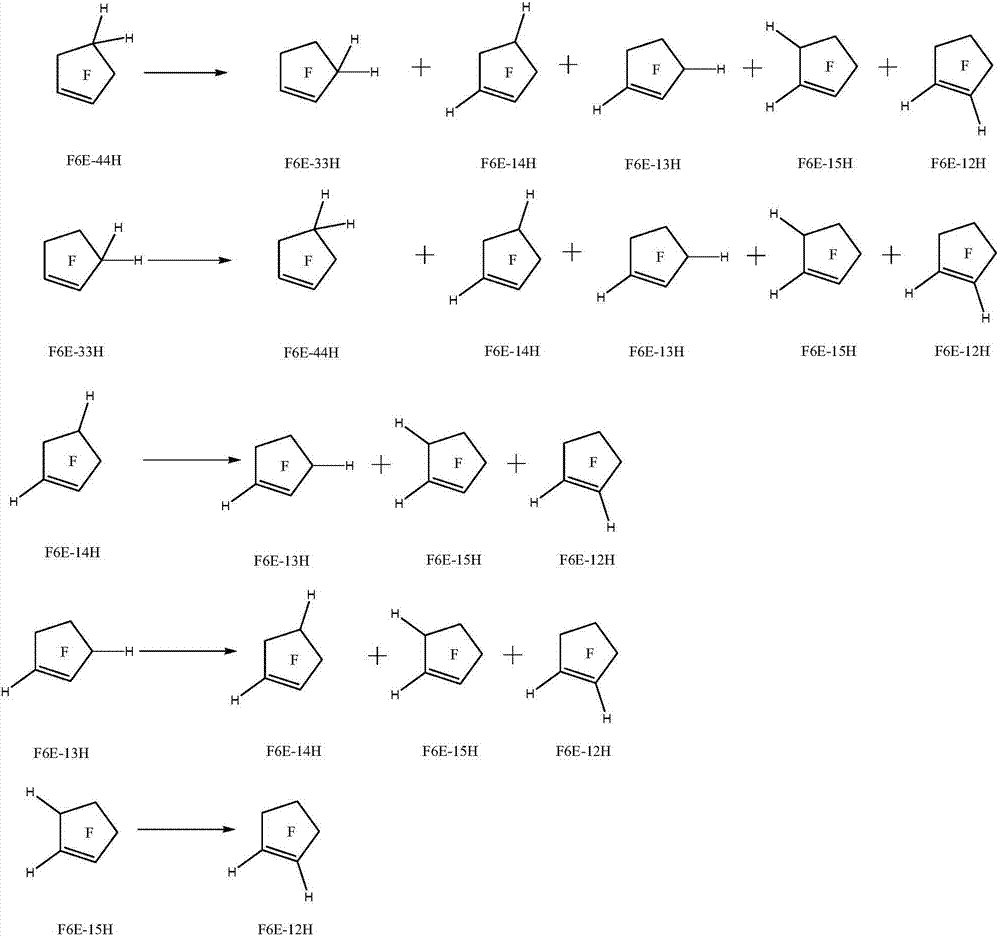
Method for preparing halogenated pentacyclic olefin by gas-phase isomerization reaction
A technology of isomerization and five-membered rings, which is applied in the field of gas-phase isomerization to prepare halogenated five-membered ring olefins, can solve the problem of harsh technology, environmental pollution and difficult control of trichloropentafluorocyclopentene isomers and other problems, to achieve the effect of cheap isomerization catalyst and easy-to-obtain raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0046] Preparation of isomerization catalyst: Dissolve metal soluble salt in water, drop concentrated ammonia water for precipitation, adjust pH value to 7.5, then age for 12 hours, wash with water, filter, dry in an oven at 80°C for 36 hours, and then Under the protection of nitrogen, calcined at 450° C. for 8 hours to obtain metal oxide, which is the precursor of metal fluoride carrier. According to the percentage composition of the alkali metal fluoride and the metal fluoride carrier, the composition is 0-10% and 10%-90%, and at a temperature of 50 ° C, the alkali metal fluoride is impregnated and supported on the metal oxide by impregnation, filtered, and heated at 80 ℃ and dried for 12 hours, the resulting solid was pulverized, tableted and formed to obtain a catalyst precursor, and 10 mL of the catalyst precursor was packed into a tube reactor made of Monel material with an inner diameter of 1 / 2 inch and a length of 30 cm, and nitrogen gas was passed into the reactor. Ro...
Embodiment 1
[0049] 10 milliliters of isomerization catalyst 100% CrF prepared by the above-mentioned method is filled in the tubular reactor made of Incon alloy with inner diameter 1 / 2 inch and long 30cm 3 . The reaction conditions are as follows: the reaction temperature is raised to 120° C., the contact time of 1,4-dichlorohexafluorocyclopentene is 5 s, and the reaction pressure is 0.1 MPa. After reacting for 100 hours, the reaction product was washed with water to separate the organic matter, and after drying to remove water, the composition of the organic matter was analyzed by gas chromatography. The reaction result was: the conversion rate of 1,4-dichlorohexafluorocyclopentene was 75.0%, 1, The selectivity of 3-dichlorohexafluorocyclopentene was 63.4%, and the selectivity of 1,2-dichlorohexafluorocyclopentene was 36.6%.
[0050] The above organic phase is rectified to obtain dichlorohexafluorocyclopentene isomer 1,4-dichlorohexafluorocyclopentene with a boiling point of 80-84°C (76...
Embodiment 2
[0052] 10 milliliters of isomerization catalysts 5% CsF / CrF prepared by the above method are filled in a tubular reactor made of Incon alloy with an inner diameter of 1 / 2 inch and a length of 30 cm 3 . The reaction conditions are as follows: the reaction temperature is raised to 400° C., the contact time of 1,4-dichlorohexafluorocyclopentene is 5 s, and the reaction pressure is 0.1 MPa. After reacting for 100 hours, the reaction product was washed with water to separate the organic matter, and after drying to remove water, the composition of the organic matter was analyzed by gas chromatography. The reaction result was: the conversion rate of 1,4-dichlorohexafluorocyclopentene was 100%, 1, The selectivity of 3-dichlorohexafluorocyclopentene was 0.7%, and the selectivity of 1,2-dichlorohexafluorocyclopentene was 99.3%.
[0053] The above organic phase was rectified to obtain 1,2-dichlorohexafluorocyclopentene, an isomer of dichlorohexafluorocyclopentene, with a boiling point o...
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com