Immunoglobulin-binding protein and affinity carrier using same
A technology of immunoglobulin and protein, which is applied in the field of immunoglobulin binding protein, can solve the problems such as improving the binding capacity of immunoglobulin of the carrier, and achieve dynamic binding capacity that is not easy to decrease, high and low binding capacity of immunoglobulin cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0101] Hereinafter, the present invention will be described in more detail with reference to examples. In addition, the following description generally shows the form of this invention, and unless otherwise indicated, this invention is not limited to this description.
reference example 1
[0102] Reference Example 1 Synthesis of Porous Particles
[0103] Dissolve 8.2 g of glycidyl methacrylate (manufactured by MITSUBISHI RAYON), 65.9 g of trimethylolpropane trimethacrylate (manufactured by Sartomer), and 90.6 g of glycerin monomethacrylate (manufactured by NOF) in To 245.8 g of 2-octanone (manufactured by Toyosei Kogyo Co., Ltd.) and 62 g of acetophenone (manufactured by Wako Pure Chemical Industries, Ltd.), 2 g of 2,2'-azobisisobutyronitrile (manufactured by Wako Pure Chemical Industries, Ltd.) was added, Prepare the organic monomer solution.
[0104] Next, 8.5 g of polyvinyl alcohol (PVA-217 manufactured by Kuraray Co., Ltd.), 0.43 g of sodium lauryl sulfate (Emal 10G manufactured by Kao Corporation), and sodium sulfate (manufactured by Wako Pure Chemical Industries, Ltd.) were added to 4240 g of pure water. 21.3 g, stirred overnight to prepare an aqueous solution.
[0105] Next, the obtained aqueous solution was poured into a 7L separable flask, a thermomet...
Embodiment 1
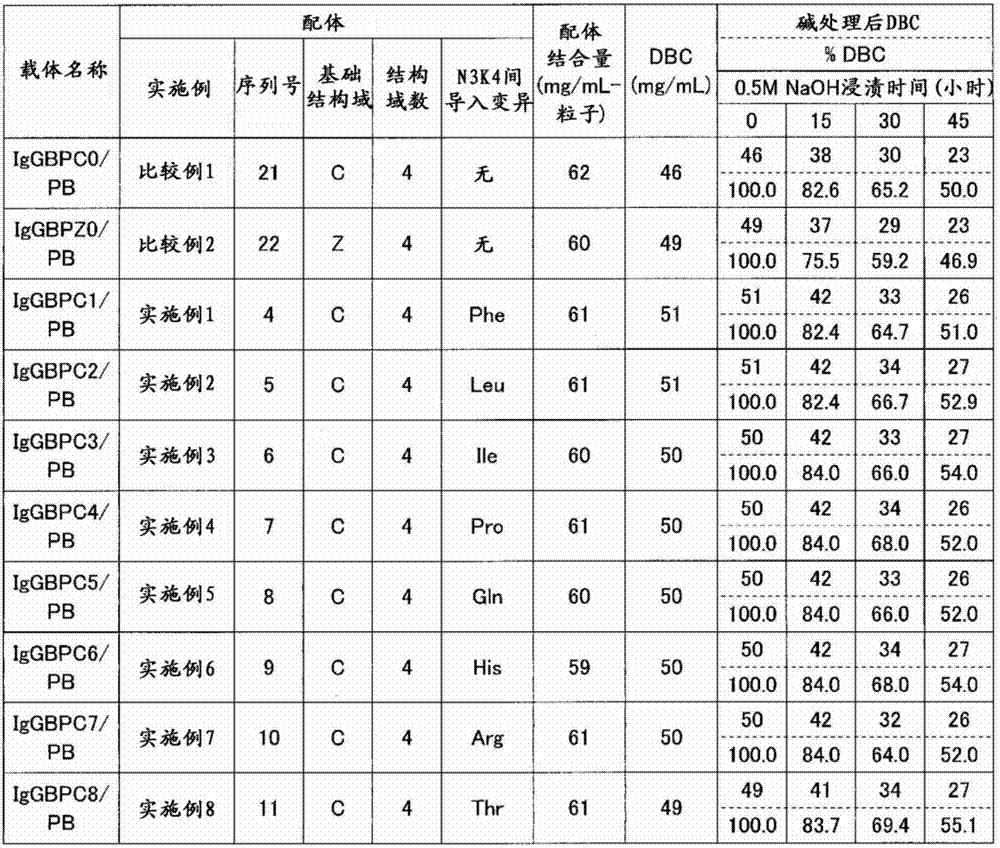
[0107] Example 1 Production of C domain recombinant immunoglobulin binding protein 1 (IgGBPC1)
[0108] A plasmid encoding the amino acid sequence shown in SEQ ID NO: 4 was prepared, and Escherichia coli competent cells BL21(DE3) (manufactured by STRATAGENE) were transformed using this plasmid to obtain a recombinant. The resulting recombinant was incubated at 37°C until the absorbance (OD600) reached about 10. Thereafter, IPTG (manufactured by Sigma-Aldrich) was added to a final concentration of 1 mM, and further incubated at 37° C. for 4 hours to express the recombinant immunoglobulin-binding protein. After protein expression, cells were recovered and disrupted in Tris buffer, pH 9.5. Immunoglobulin-binding proteins were purified from the obtained cell lysates by anion-exchange chromatography (Q-Sepharose FF, manufactured by GE Health Care Bio-Sciences) and cation-exchange chromatography (SP-Sepharose FF, manufactured by GE Health Care Bio-Sciences). Purified immunoglobuli...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com