Method for chiral preparation of (S)-tetrahydro-1-naphthoic acid and derivative thereof
A derivative, naphthoic acid technology, applied in the field of pharmaceutical chemical synthesis, can solve the problems of harsh reaction conditions, high safety risks, environmental pollution, etc., and achieve the effect of simple operation and high atom economy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
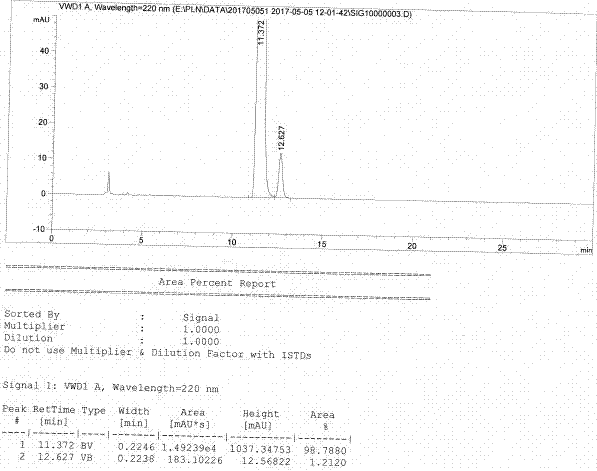
Image
Examples
Embodiment 1
[0031] Embodiment 1: ( S )-1,2,3,4-tetrahydro-1-naphthoic acid preparation
[0032] Add 500 ml of toluene and 100 g of 1-naphthoic acid into a 1L three-necked flask, stir and cool down to below 10°C. Add dropwise 84 ml of thionyl chloride and 1 ml N,N -dimethylformamide. Heat up to 40 °C and stir for 4 h. The reaction solution was concentrated to obtain 115 g of yellow oil. Under stirring, add 200 ml of toluene, 115 g of the above-mentioned yellow oil, and 160 ml of triethylamine into a 2 L three-necked flask, and cool to 10°C. drop ( R )-(+)-Ethyl 2-hydroxypropionate toluene solution (68.6 g dissolved in 300 ml toluene), raised to room temperature and stirred for 2 hours. Slowly add 400 ml of 10% citric acid aqueous solution dropwise, and let stand to separate layers. The toluene layer was washed with 100 ml of saturated aqueous sodium chloride solution. The toluene layer was dried over anhydrous sodium sulfate. Filter and wash the filter cake with toluene. The fil...
Embodiment 2
[0035] Embodiment 2: ( S )-1,2,3,4-tetrahydro-6-methoxyl-1-naphthoic acid preparation
[0036] Add 250 ml of toluene and 50 g of 6-methoxy-1-naphthoic acid into a 1L three-necked flask, stir and cool down to below 10°C. Add dropwise 32 ml of thionyl chloride and 1 ml N,N -dimethylformamide. Heat up to 40 °C and stir for 4 h. The reaction solution was concentrated to obtain 54 g of yellow oil. Under stirring, add 100 ml of toluene, 54 g of the above yellow oil, and 80 ml of triethylamine into a 2 L three-necked flask, and cool to 10°C. drop ( R )-(+)-2-Hydroxypropionic acid ethyl ester 29.2 g was dissolved in 150 ml of toluene solution, raised to room temperature and stirred for 2 hours. Slowly add 200 ml of 10% citric acid aqueous solution dropwise, and let stand to separate layers. The toluene layer was washed with 50 ml of saturated aqueous sodium chloride solution. The toluene layer was dried over anhydrous sodium sulfate. Filter and wash the filter cake with tolu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com