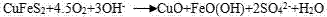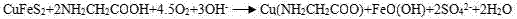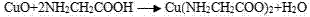Method for leaching Cu from alkaline glycinate solution
A technology of glycinate and glycine, which is applied in the field of metallurgy, can solve the problems of high acid consumption and affect the leaching efficiency, and achieve the effect of cheap medicine, simple operation and avoiding high acid consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] A method for leaching copper with alkaline glycinate solution includes the following steps:
[0017] (1) Add 10g chalcopyrite (all finely ground to -45μm) in a 1L stirring tank, make slurry, add 0.12mol / L glycine solution, add NaOH solution to adjust the pH to 10, at room temperature (about 23°C) Continue stirring for 8h, and the stirring intensity is 300r / min. Among them, the pulp concentration is 1%, and the glycine concentration after mixing is about 0.1mol / L (the water required for pulping mainly comes from glycine solution and NaOH solution. The amount of NaOH solution added is small, so the concentration of glycine solution is not greatly reduced);
[0018] (2) Filter the mixed solution after leaching in step (1), add NaS to the filtrate, stir and filter to obtain CuS precipitate;
[0019] (3) The most calculated copper leaching rate in chalcopyrite is 40.5%;
[0020] The CuS precipitate undergoes subsequent conventional reduction treatment to obtain copper.
Embodiment 2
[0022] A method for leaching copper with alkaline glycinate solution includes the following steps:
[0023] (1) Add 20g chalcopyrite (all finely ground to -37μm) in a 1L stirring tank, make slurry, add 0.21mol glycine solution, add NaOH solution to adjust the pH to 10.5, the concentration of the resulting ore slurry is 20%, after slurry adjustment The glycine concentration is about 0.2mol / L, keep the slurry temperature at 30℃, continue to stir for 12h, and the stirring intensity is 300r / min. Similarly, the water required for pulping mainly comes from glycine solution and NaOH solution. The amount of NaOH solution added is small, so the concentration of glycine solution is not reduced greatly;
[0024] (2) Filter the mixed solution after leaching in step (1), add NaS to the filtrate, stir and filter to obtain CuS precipitate;
[0025] (3) The most calculated copper leaching rate in chalcopyrite is 60.3%;
[0026] The CuS precipitate undergoes subsequent conventional reduction treatmen...
Embodiment 3
[0028] A method for leaching copper with alkaline glycinate solution includes the following steps:
[0029] (1) Add 40g chalcopyrite (all finely ground to -25μm) in a 1L stirring tank, and make pulp; add glycine to NaOH solution to make alkaline glycinate solution, and add the prepared alkaline glycinate solution to the slurry Here, adjust the slurry pH to 11, the resulting slurry concentration is 5%, the glycine concentration after slurry adjustment is 0.3mol / L, keep the slurry temperature at 40℃, continue stirring for 18h, and the stirring intensity is 200r / min. The source of the water needed in the slurry is the NaOH solution and the added water, and the amount of additional water needed to be adjusted according to the concentration of the NaOH solution;
[0030] (2) Filter the mixed solution after leaching in step (1), add NaS to the filtrate, stir and filter to obtain CuS precipitate;
[0031] (3) The most calculated copper leaching rate in chalcopyrite is 65.7%;
[0032] The Cu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com