Method for measuring effective ingredients in Huoxiang Zhengqi oral liquid
A technology for Zhengqi oral liquid and active ingredients, which is applied in the field of component content determination in Huoxiang Zhengqi oral liquid, can solve the problems of poor versatility and reproducibility, unsafe toxic reagents, different mobile phases, etc., and achieves good separation, Avoid the harm of human body and the environment, and the effect of short detection time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] A method for determining active ingredients in Huoxiangzhengqi Oral Liquid (method for simultaneous determination of hesperidin / magnokiol / honokiol content)
[0044] Preparation of standard reference substance solution: Accurately weigh hesperidin, magnolol and honokiol standard reference substance, prepare the mixed reference substance solution shown in Table 1, filter through a 0.45 μm filter membrane, and set aside.
[0045] Table 1 Reference substance solution
[0046]
[0047] Preparation of the test solution: Accurately measure 5.0 mL of Huoxiang Zhengqi Oral Liquid stock solution and put it into a 10 mL volumetric flask, dilute to the mark with methanol, mix and filter with a 0.45 μm membrane filter, and set aside.
[0048] (1) System adaptability test
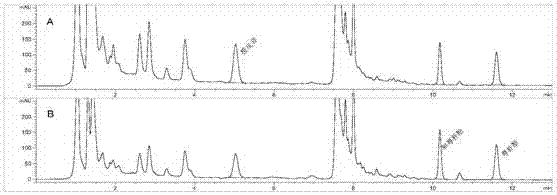
[0049] Take the reference substance solution and the test solution, and the chromatographic conditions are: (1) Chromatographic column: Waters Xbridge C18, 3.5 μm, 4.6×100mm; (2) Column temperature: 35°C; (3) ...
Embodiment 2
[0076] Parameter optimization
[0077] (1) Preparation method of the test article
[0078] The stock solution of the test sample is diluted 1 time, 2 times, 2.5 times, 5 times and no dilution with methanol or methanol to obtain 5 kinds of test products, which are analyzed according to the chromatographic conditions under the system suitability test. The results show that hesperidin, There was no significant difference in the contents of honokiol and magnolol, indicating that the solvent and dilution factor would not affect the test results. Water, 20%, 40%, 60%, 80% and 100% methanol and ethanol are respectively diluted twice for the test product, and analyzed according to the chromatographic conditions under the system suitability test, the results show that when the concentration of methanol and ethanol is low At 60%, compared with the test product stock solution and 100% methanol or ethanol dilution measurement results, the contents of hesperidin, honokiol and magnolol wer...
Embodiment 3
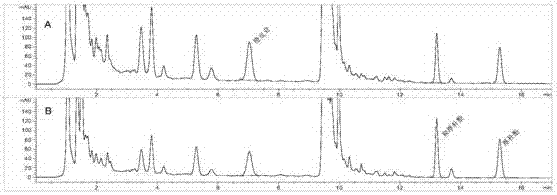
[0126] For 21 different batches of samples (provided by Taiji Group Fuling Pharmaceutical Factory Co., Ltd., batch numbers are shown in Table 21), the method of the present invention and the method of "Chinese Pharmacopoeia (2015)" were used to measure orange peel in Huoxiangzhengqi Oral Liquid respectively. The contents of glycosides, honokiol and magnolol were determined respectively. According to the data obtained from the analysis, it is judged whether there is a significant difference between the two methods. Follow the steps below for analysis.
[0127] Preparation of standard solution: Accurately weigh hesperidin, honokiol and magnolol standard reference substance, after diluting with methanol, prepare 2 parts of mixed reference substance solutions with different concentrations, as shown in Table 19:
[0128] Table 19 Reference substance solution
[0129]
[0130] 1, adopt the method analysis of the present invention (marked as method A)
[0131] 1) Preparation of...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com