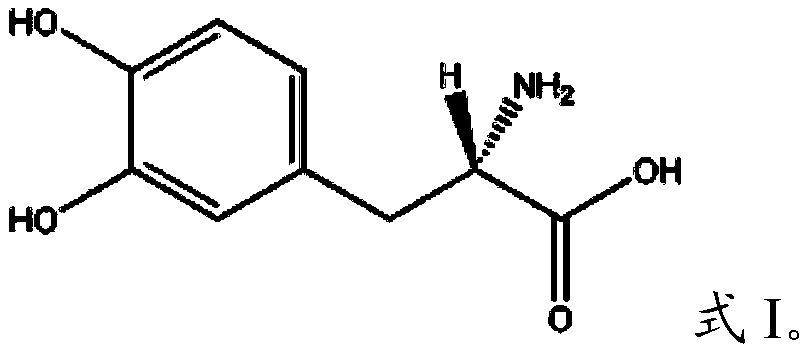
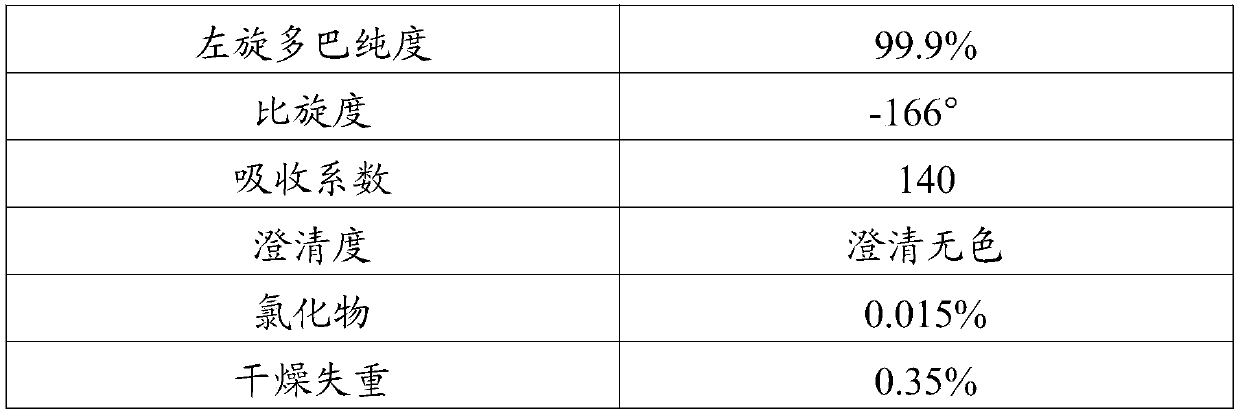
A kind of separation and purification method of levodopa
A technology for separation, purification and levodopa, which is applied in chemical instruments and methods, organic chemistry, cyanide reaction preparation, etc., can solve problems such as low yield of levodopa products, and achieves reduction of possibility, improvement of yield, and improvement of The effect of the amount of precipitation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0071] Preparation of raw material liquid containing levodopa:
[0072] First prepare the substrate solution: mix catechol 10g, sodium pyruvate 12g, ammonium acetate 45g, sodium sulfite 2g, ethylenediaminetetraacetic acid 1g, pyridoxal phosphate 0.1g with 720mL pure water, heat to 20°C, Stir to dissolve.
[0073] Add ammonia water to the above substrate solution, adjust the pH value to 8.15, set the volume to 900ml, add 100mL of commercially available tyrosine phenol lyase crude enzyme solution with a mass concentration of 5%, then seal the reaction vessel and fill it with nitrogen , at 15°C, 200rpm shaker or stirring conditions for biocatalytic reaction.
[0074] In addition to 0min, sampling and determination of the residual amount of catechol and sodium pyruvate every hour, when the content of catechol and sodium pyruvate is low, then add an appropriate amount of catechol and sodium pyruvate, so that The concentration of hydroquinone is 6g / L, and the concentration of sodi...
Embodiment 2
[0095] The substrate solution was prepared according to the method described in Example 1, and a raw material solution with a levodopa content of 70 g / L was obtained through catalysis by tyrosine phenol lyase. Getting levodopa content is 500L of raw material liquid of 70g / L, and the separation and purification of this raw material liquid comprises the following steps:
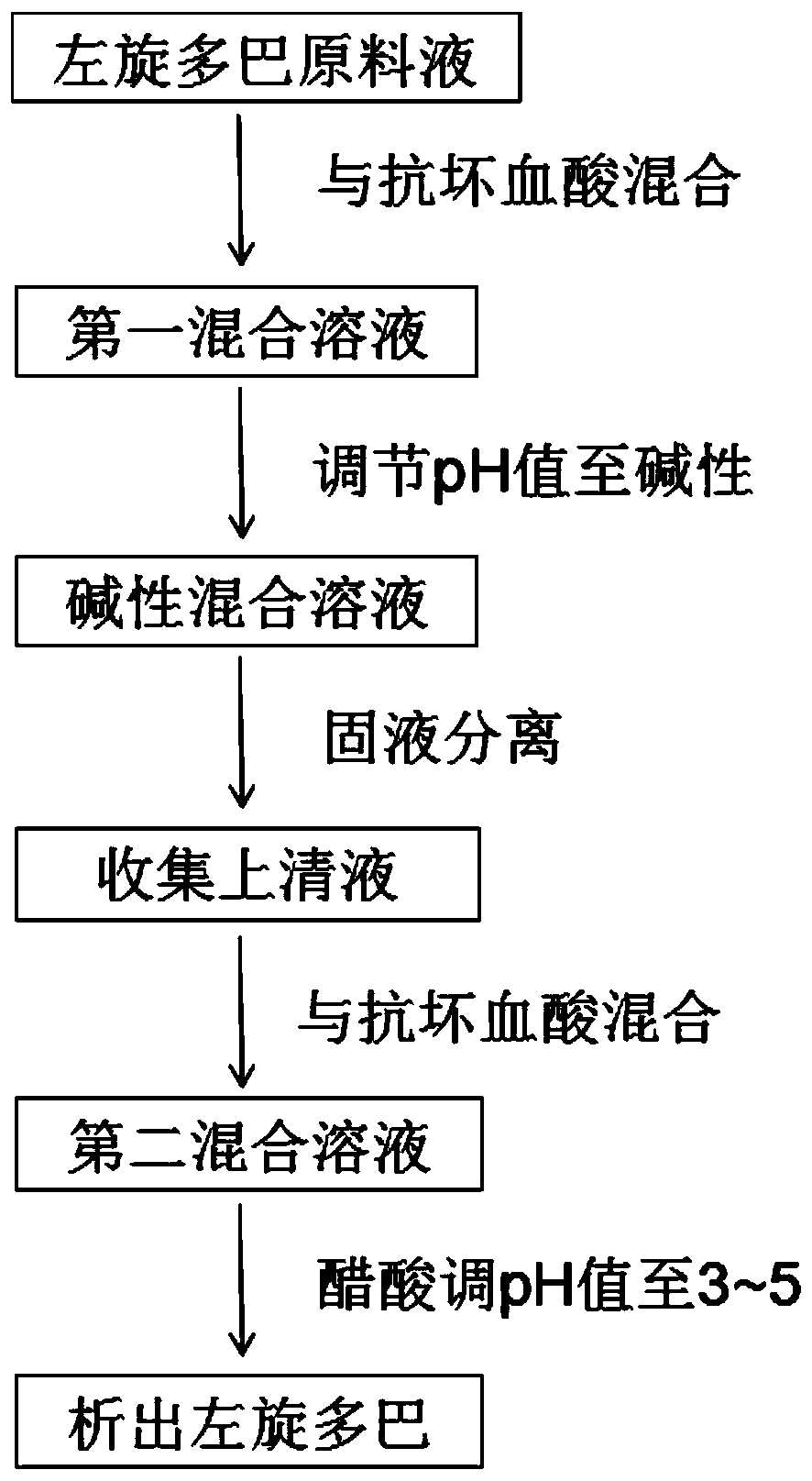
[0096] 1) Add 500g of ascorbic acid into the raw material solution, stir evenly, and fully dissolve;
[0097] 2) Under the condition of rapid stirring of the raw material solution at a speed of 300rpm, slowly add a potassium hydroxide solution with a mass-volume concentration of 350g / L, adjust the pH value of the solution to 12.5, and obtain an alkaline mixed solution; the addition of the potassium hydroxide The speed is: 20mL / min;
[0098] 3) The alkaline mixed solution obtained in step 2) was subjected to low-temperature high-speed centrifugation at 5° C. and a rotational speed of 4500 rpm for 30 minutes, an...
Embodiment 3
[0114] Get 500L of raw material liquid containing levodopa, and the concentration of levodopa in the raw material liquid is 50g / L. The separation and purification of this raw material liquid comprises the following steps:
[0115] 3) Add 500g of ascorbic acid into the raw material solution, stir evenly, and fully dissolve;
[0116] 4) Under the condition of rapid stirring of the raw material solution at a speed of 400rpm, slowly add a sodium hydroxide solution with a mass-volume concentration of 380g / L, adjust the pH value of the solution to 13, and obtain an alkaline mixed solution; the addition of the sodium hydroxide The speed is: 25mL / min;
[0117] 3) The alkaline mixed solution obtained in step 2) was subjected to low-temperature high-speed centrifugation at 4°C and a rotational speed of 4000 rpm for 20 minutes, and the supernatant was collected, totaling 500 L;
[0118] 4) Add 15 kg of powdered activated carbon to the supernatant obtained in step 3), heat up to 40° C.,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com