Preparation method and application of hypochloric acid-responsive fluorescence sensing material
A technology of fluorescent sensing and hypochlorous acid, which is applied in the field of chemical fluorescent sensing materials, can solve problems such as unsatisfactory detection limit and low fluorescence quantum yield, and achieve sensitive and selective recognition performance, large optical performance, and detection low limit effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Example 1: Preparation of hypochlorous acid-responsive fluorescent chemical sensing materials
[0032] Weigh 0.81g of 6-carboxycoumarin and 0.92g of thiosemicarbazide into a round bottom flask, add 5mL of anhydrous methanol to dissolve, and heat at 80°C for 4h. Filtrate while hot, recrystallize the filtrate, filter with suction, and wash with 5 mL of anhydrous methanol three times to obtain a white solid.
Embodiment 2
[0033] Example 2: Preparation of hypochlorous acid-responsive fluorescent chemical sensing materials
[0034] Weigh 1.19g of 6-carboxycoumarin and 1.84g of thiosemicarbazide into a round bottom flask, add 15mL of anhydrous methanol to dissolve, and heat at 100°C for 6h. Filtrate while hot, recrystallize the filtrate, filter with suction, and wash with 15 mL of anhydrous methanol three times to obtain a white solid.
Embodiment 3
[0035] Example 3: Preparation of hypochlorous acid-responsive fluorescent chemical sensing materials
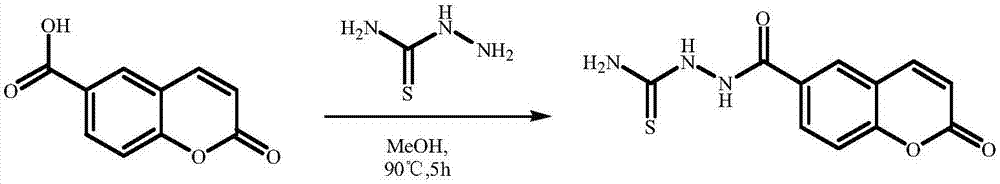
[0036] Weigh 1g of 6-carboxycoumarin and 1.38g of thiosemicarbazide into a round bottom flask, add 10mL of anhydrous methanol to dissolve, and heat at 90°C for 5h. Filtrate while hot, recrystallize the filtrate, filter with suction, and wash with 10 mL of anhydrous methanol three times to obtain a white solid.
[0037] Such as figure 1 Shown is a schematic diagram of the synthesis process of the hypochlorous acid-responsive fluorescent sensing material.
[0038] Such as figure 2 Shown is the infrared spectrum of the fluorescent sensing material. FT-IR (KBr, cm -1 ):3369,3264,3178,1745,1684,1645,1618,1568,1532,1421,1374,1317,1286,1227,1207,1165,999,802,770,647. The functional group of the fluorescent sensing material can be determined by the infrared spectrum.
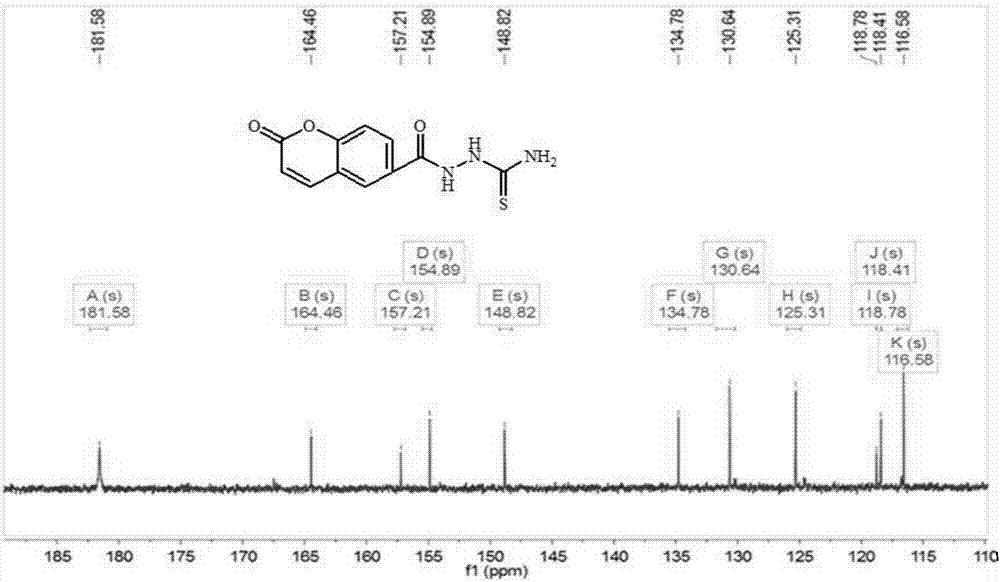
[0039] Such as image 3 and Figure 4 Shown are the fluorescent sensing materials 1 H NMR and 13 C NMR im...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com