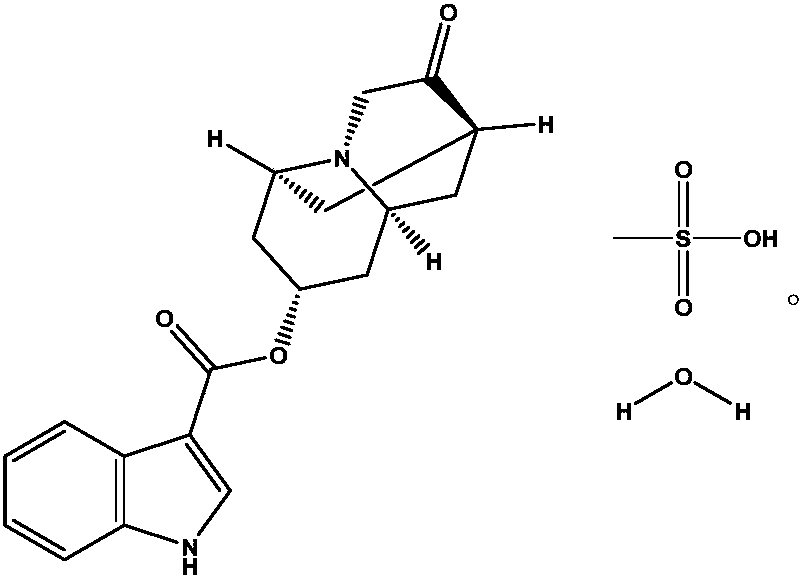
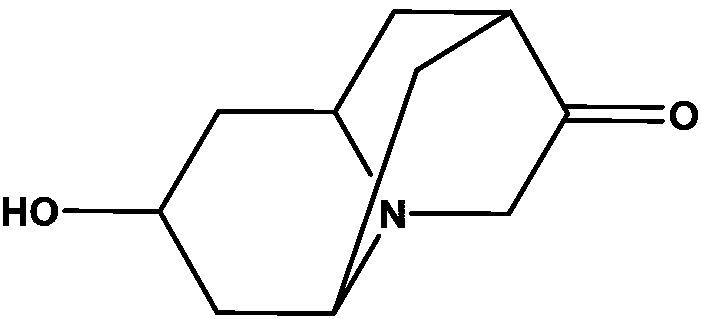
Injection composition containing dolasetron mesylate
A technology of dolasetron mesylate and a composition, applied in the field of medicine, can solve problems such as reducing the quality of dolasetron preparations, and achieve the effects of enhancing storage stability, simple method and inhibiting degradation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0098] Example 1 Preparation of 12.5mg / 0.625ml Dolasetron Mesylate Injection
[0099] .
[0100] The preparation method is as follows:
[0101] 1) Take dolasetron mesylate crude drug, pulverize it into fine powder, and set aside;
[0102]2) Take 80% of the total volume of water for injection, dissolve sodium glutamate, glycerin, and 0.1M HCl in sequence to adjust the pH value of the solution to 3.0-4.0;
[0103] 3) Take the solution obtained in step 2), add the dolasetron mesylate bulk drug powder obtained in step 1), stir, and dissolve to obtain the drug-containing solution ①;
[0104] 4) Take the drug-containing solution ① obtained in step 3), add water for injection to the full amount, and adjust the pH value to 3.0-4.0 with 0.1M HCl or NaOH aqueous solution to obtain the drug-containing solution ②;
[0105] 5) Take the drug-containing solution ② obtained in step 4), adsorb the pyrogen with 0.2% activated carbon, and filter to obtain the drug-containing solution ③;
...
Embodiment 2
[0108] Example 2 Preparation of 100mg / 5ml Dolasetron Mesylate Injection
[0109] .
[0110] The preparation method is as follows:
[0111] 1) Take dolasetron mesylate crude drug, pulverize it into fine powder, and set aside;
[0112] 2) Take 80% of the total volume of water for injection, dissolve sodium glutamate, glycerin, and 0.1M HCl in sequence to adjust the pH value of the solution to 3.0-4.0;
[0113] 3) Take the solution obtained in step 2), add the dolasetron mesylate bulk drug powder obtained in step 1), stir, and dissolve to obtain the drug-containing solution ①;
[0114] 4) Take the drug-containing solution ① obtained in step 3), add water for injection to the full amount, and adjust the pH value to 3.0-4.0 with 0.1M HCl or NaOH aqueous solution to obtain the drug-containing solution ②;
[0115] 5) Take the drug-containing solution ② obtained in step 4), adsorb the pyrogen with 0.2% activated carbon, and filter to obtain the drug-containing solution ③;
[011...
Embodiment 3
[0118] Example 3 Preparation of 500mg / 25ml Dolasetron Mesylate Injection
[0119] .
[0120] The preparation method is as follows:
[0121] 1) Take dolasetron mesylate crude drug, pulverize it into fine powder, and set aside;
[0122] 2) Take 80% of the total volume of water for injection, dissolve sodium glutamate, glycerin, and 0.1M HCl in turn to adjust the pH value of the solution to 3.0-4.0; 3) Take the solution obtained in step 2) and add the obtained methanesulfonic acid in step 1) Lasetron bulk drug powder, stirred, dissolved, to obtain the drug-containing solution ①;
[0123] 4) Take the drug-containing solution ① obtained in step 3), add water for injection to the full amount, and adjust the pH value to 3.0-4.0 with 0.1M HCl or NaOH aqueous solution to obtain the drug-containing solution ②;
[0124] 5) Take the drug-containing solution ② obtained in step 4), adsorb the pyrogen with 0.2% activated carbon, and filter to obtain the drug-containing solution ③;
[0...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com