Separation and detection method of related substances in ophthalmic gel
A technology for related substances and ophthalmic gels, which is applied in the field of separation and detection of related substances in ophthalmic gels, can solve problems such as adverse effects on safety and effectiveness, complex components, etc., to ensure safety and quality. Properties, short detection time, good column efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
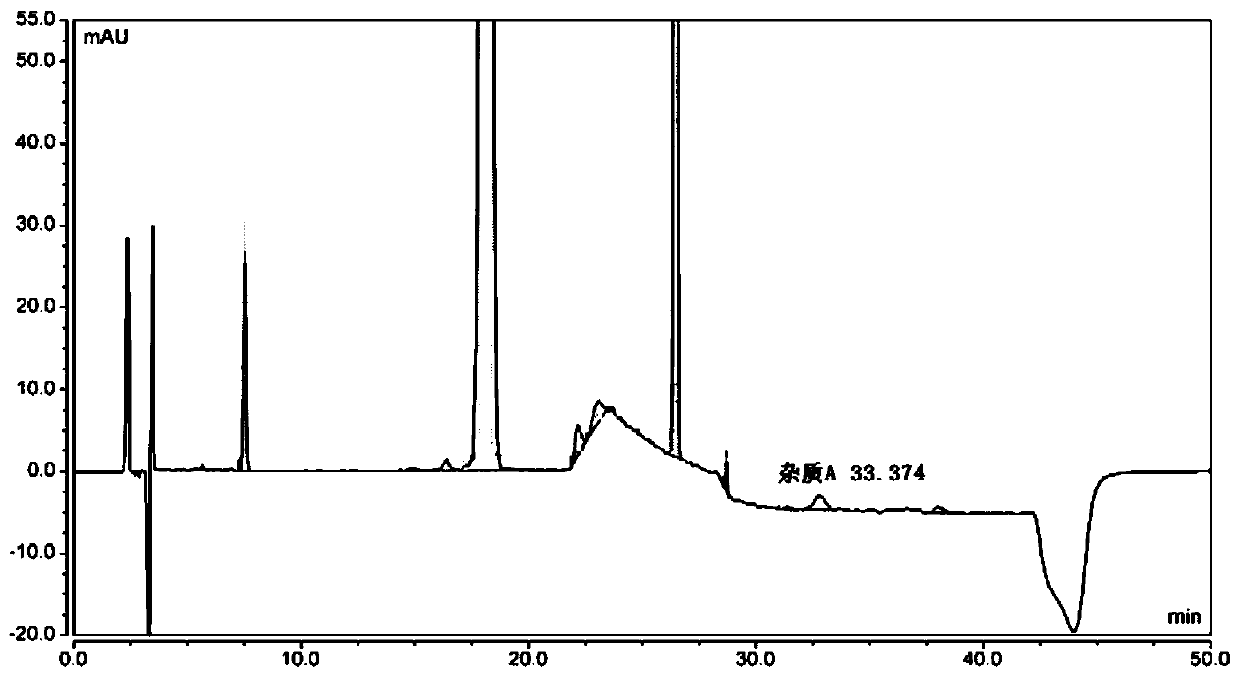
[0073] Dilute the levofloxacin hydrochloride ophthalmic gel with 0.1 mol / L hydrochloric acid to a concentration of 1 mg / mL, centrifuge at 3000 r / min for 15 min, and take the supernatant as the analyte solution.
[0074] The hydroxybenzoic acid reference substance and the ciprofloxacin reference substance are respectively diluted with water to obtain the hydroxybenzoic acid reference solution and the ciprofloxacin reference solution. Dilute the impurity E reference substance with 0.1 mol / L hydrochloric acid to a concentration of 5 μg / mL to obtain the impurity E reference solution. Dilute the impurity A reference substance with 6 mol / L ammonia water and water to a concentration of 0.15 mg / mL to obtain the impurity A reference solution.
[0075] Take 10 μL of the analyte solution, the hydroxybenzoic acid reference solution, the impurity E reference solution, the ciprofloxacin reference solution and the impurity A reference solution, respectively, inject 10 μL into the Dionex U300...
Embodiment 2
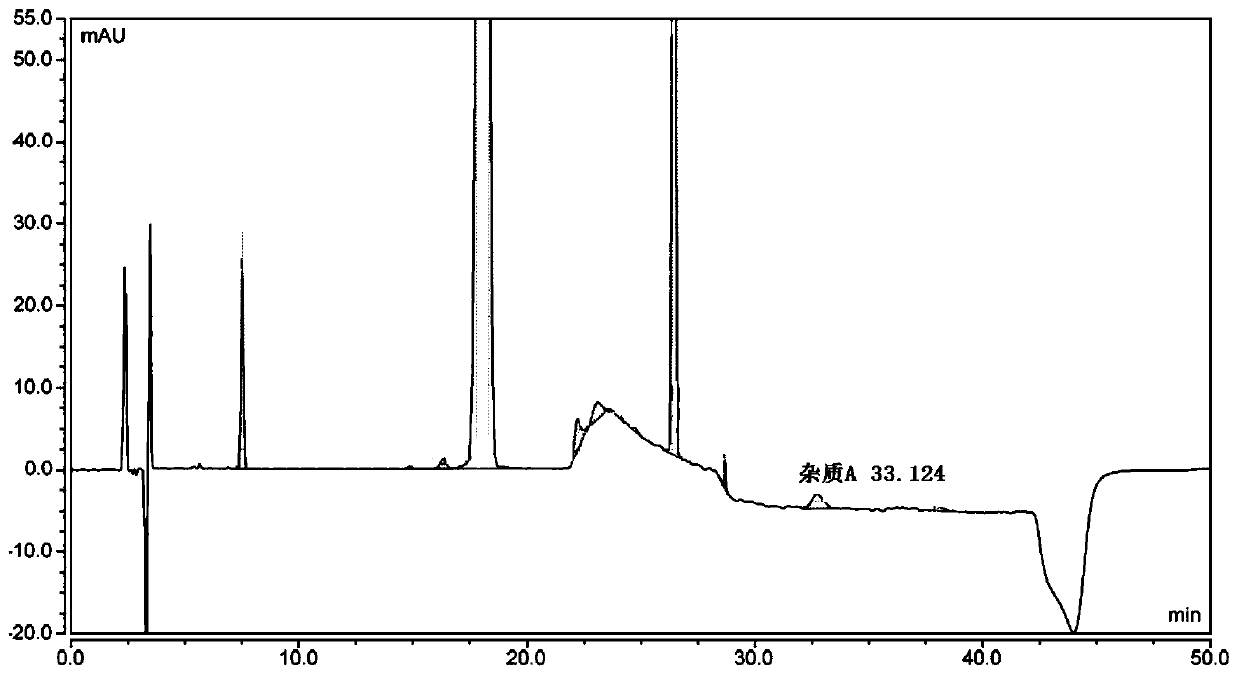
[0086] Dilute the levofloxacin hydrochloride ophthalmic gel with 0.05 mol / L hydrochloric acid to a concentration of 0.5 mg / mL, centrifuge at 2500 r / min for 20 min, and take the supernatant as the solution to be tested.
[0087] The hydroxybenzoic acid reference substance and the ciprofloxacin reference substance are respectively diluted with water to obtain the hydroxybenzoic acid reference solution and the ciprofloxacin reference solution. Dilute the impurity E reference substance with 0.08mol / L hydrochloric acid to a concentration of 4.5 μg / mL to obtain the impurity E reference solution. Dilute the impurity A reference substance with 5 mol / L ammonia water and water to a concentration of 0.1 mg / mL to obtain the impurity A reference solution.
[0088] Take 15 μL of the test substance solution, the hydroxybenzoic acid reference solution, the impurity E reference solution, the ciprofloxacin reference solution and the impurity A reference solution, respectively, inject 15 μL into...
Embodiment 3
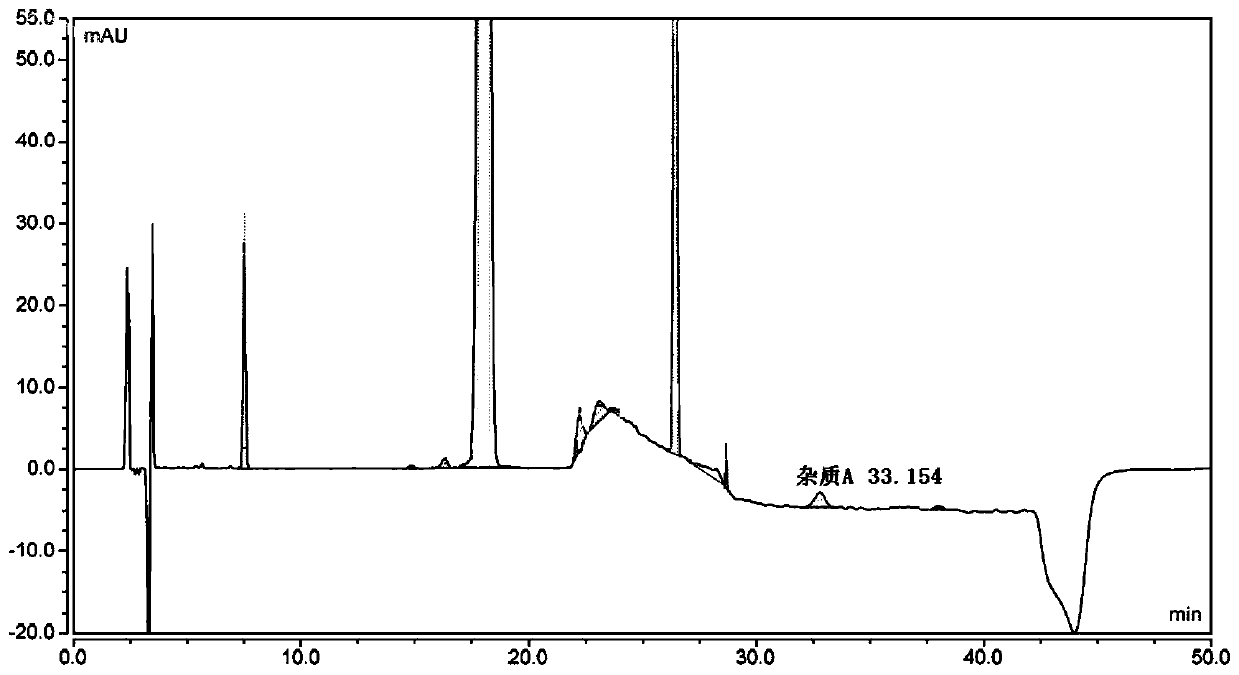
[0099] Dilute the levofloxacin hydrochloride ophthalmic gel with 0.5mol / L hydrochloric acid to a concentration of 1.5mg / mL, centrifuge at 3500r / min for 10min, and take the supernatant as the analyte solution.
[0100] The hydroxybenzoic acid reference substance and the ciprofloxacin reference substance are respectively diluted with water to obtain the hydroxybenzoic acid reference solution and the ciprofloxacin reference solution. Dilute the impurity E reference substance with 0.12mol / L hydrochloric acid to a concentration of 5.5 μg / mL to obtain the impurity E reference solution. Dilute the impurity A reference substance with 7 mol / L ammonia water and water to a concentration of 0.2 mg / mL to obtain the impurity A reference solution.
[0101] Take respectively 20 μL of test substance solution, hydroxybenzoic acid reference solution, impurity E reference solution, ciprofloxacin reference solution and impurity A reference solution, inject into Waters Alliance2695 high performance...
PUM
| Property | Measurement | Unit |
|---|---|---|
| volume | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com