Application of malt1 targeting inhibitor in the preparation of malt1-dependent tumor therapy drugs
A dependence and tumor technology, applied in the field of biomedicine, can solve problems such as off-target effects, low cell permeability, and limited development and utilization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
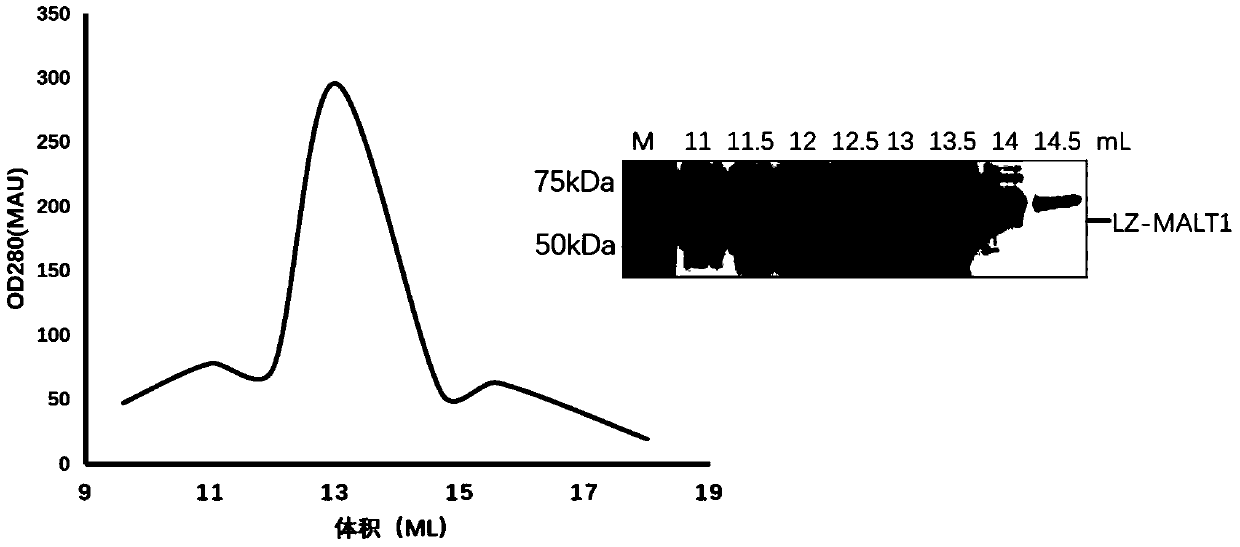
[0046] Embodiment 1, LZ-MALT1 protein expression and purification
[0047] Because the full-length MALT1 and its caspase-like domain (340-789 amino acids) exist as monomers in physiological solution, the enzymatic cleavage activity is low, and MALT1 needs to form a dimer to better exert the enzymatic cleavage activity. After analysis and research, the inventors fused the caspase-like domain (340-789) fragment of MALT1 with the leucine zipper dimer fragment to construct an in vitro recombinant protein of LZ-MALT1.
[0048] The amino acid sequence of MALT1 (340-789) is as follows (SEQ ID NO: 1):
[0049] AKDKVALLIGNMNYREHPKLKAPLVDVYELTNLLRQLDFKVVSLLDLTEYEMRNAVDEFLLLLDKGVYGLLYYAGHGYENFGNSFMVPVDAPNPYRSENCLCVQNILKLMQEKETGLNVFLLDMCRKRNDYDDTIPILDALKVTANIVFGYATCQGAEAFEIQHSGLANGIFMKFLKDRLLEDKKITVLLDEVAEDMGKCHLTKGKQALEIRSSLSEKRALTDPIQGTEYSAESLVRNLQWAKAHELPESMCLKFDCGVQIQLGFAAEFSNVMIIYTSIVYKPPEIIMCDAYVTDFPLDLDIDPKDANKGTPEETGSYLVSKDLPKHCLYTRLSSLQKLKEHLVFTVCLSYQYSGLEDTVEDKQEVNVGKPLIAKLDMHRG...
Embodiment 2
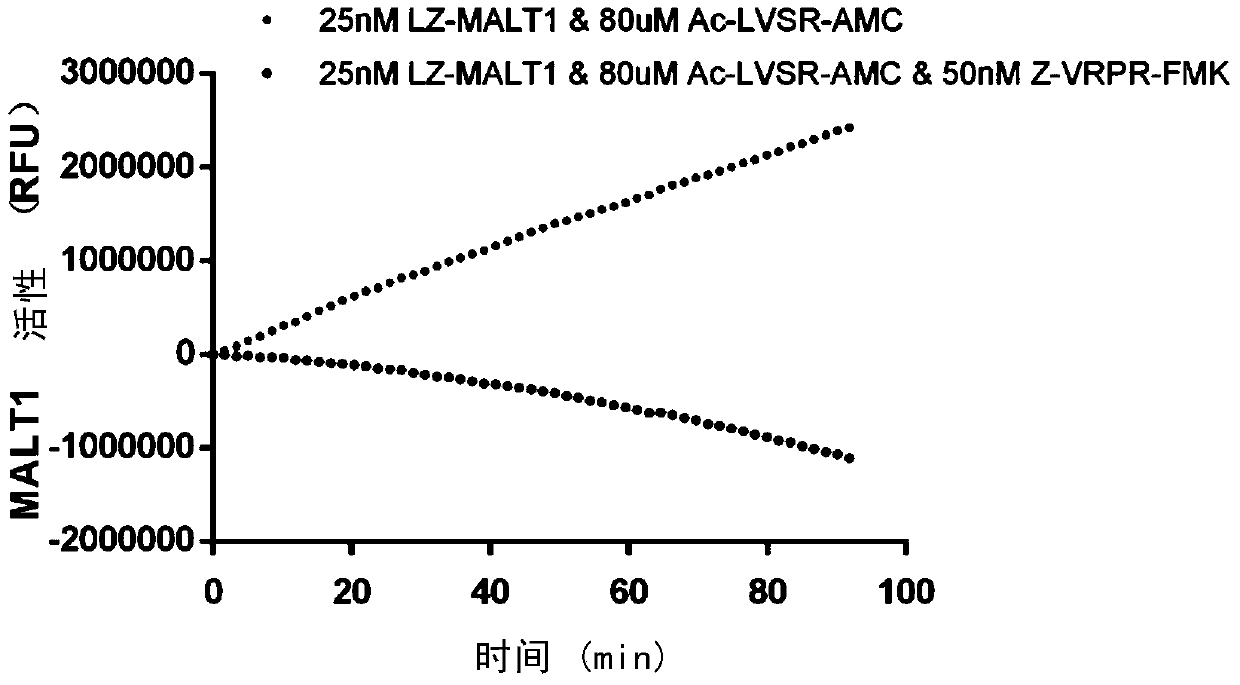
[0056] Example 2, High-throughput screening of LZ-MALT1-specific small molecule inhibitors
[0057]The screening system established by the present inventors includes LZ-MALT1 and Ac-LVSR-AMC, wherein Ac-LVSR-AMC is the substrate of MALT1, MALT1 can recognize and cut the LVSR site, and the released AMC group can emit Fluorescence, so the enzymatic activity of MALT1 can be characterized by the detected fluorescence value. For determining the optimal high-throughput screening reaction conditions, the inventors set a series of LZ-MALT1 concentrations (25nM, 50nM, 100nM, 200nM, etc.) and substrate concentrations (62.5uM, 125uM, 250uM, etc.), and did two Dimensional orthogonal experiment, detect and record the fluorescence value every 30 seconds, the results are as follows figure 2 As shown, the fluorescence value has a linear relationship with time. As the substrate concentration increases, the reaction signal increases, and as the enzyme concentration increases, the reaction sig...
Embodiment 3
[0068] Embodiment 3, the effect verification of compound
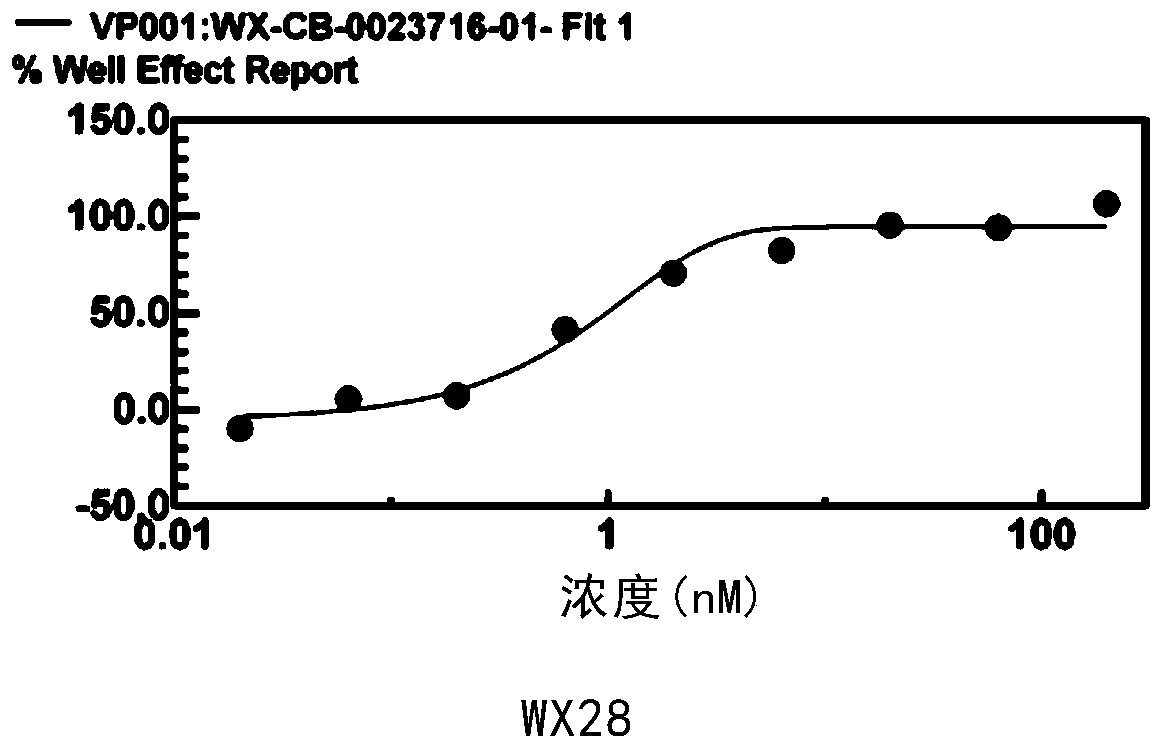
[0069] For the small molecular compounds screened in Example 2, the effects of these compounds were verified by dose-response experiments, with the half inhibitory concentration (IC50) being less than 20 μM and having a good dose-response curve as the standard.
[0070] From these compounds, a small molecular compound with strong inhibitory effect, S-type dose-response curve, and IC50 less than 1nM was obtained as a MALT1 inhibitor, such as image 3 and Table 2.
[0071] Table 2
[0072]
[0073] The compounds in Table 2 can effectively inhibit the activity of MALT1 in vitro, are effective MALT1 inhibitors, and can be used as targeted drugs for the clinical treatment of ABC-DLBCL.
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com