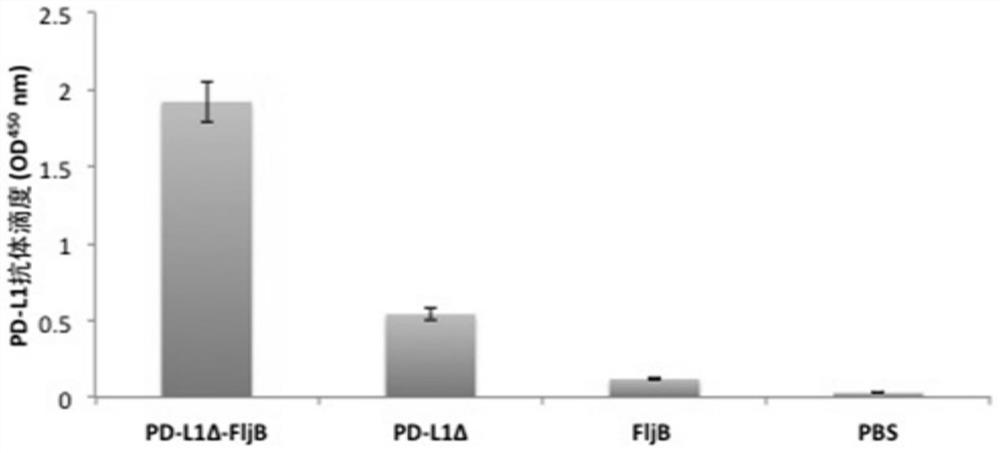
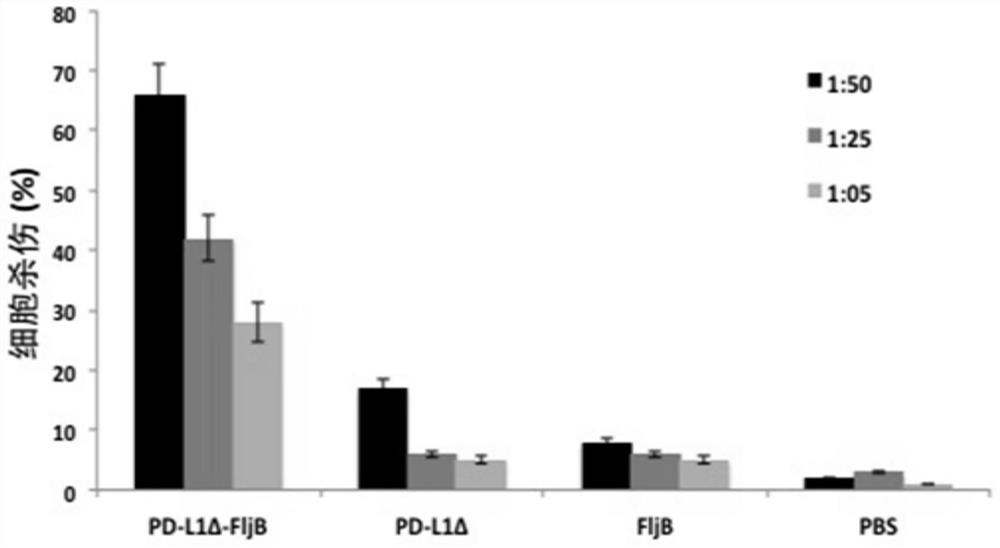
Anti-immune checkpoint PD-L1 and PD-L2 tumor vaccines
An immune checkpoint and immune stimulation technology, applied in the field of bioengineering, can solve the problems of weak clinical efficacy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0095] In the following examples, the materials and methods used are as follows:
[0096] Preparation of dendritic (DC) cells
[0097]The method for isolating DC cells from mouse bone marrow is as follows: the bone marrow is flushed from the limbs of the mouse, passed through a nylon mesh, and red blood cells are removed with ammonium chloride. The cells were then fully rinsed with RPMI-1640 medium, and then cultured in 2.5ml RPMI-1640 medium containing 10% FBS, 20ng / ml recombinant mouse GM-CSF (rmGM-CSF) and 20ng / ml recombinant Mouse IL-4 (rmIL-4) (purchased from PeproTech, Inc., Rocky Hill, NJ). On day 2 and day 4 of the culture process, the cell supernatant was removed and replaced with fresh medium containing 20 ng / ml rmGM-CSF and 20 ng / ml rmIL-4. Cells were cultured in an incubator at 37°C, 5% CO2. At 48 hours of the culture process, non-adherent granulocytes were removed and fresh medium was replaced. After the cells were cultured for 7 days, by FACS analysis, more t...
Embodiment 2
[0102] Example 2 Construction of human fusion protein expression vector
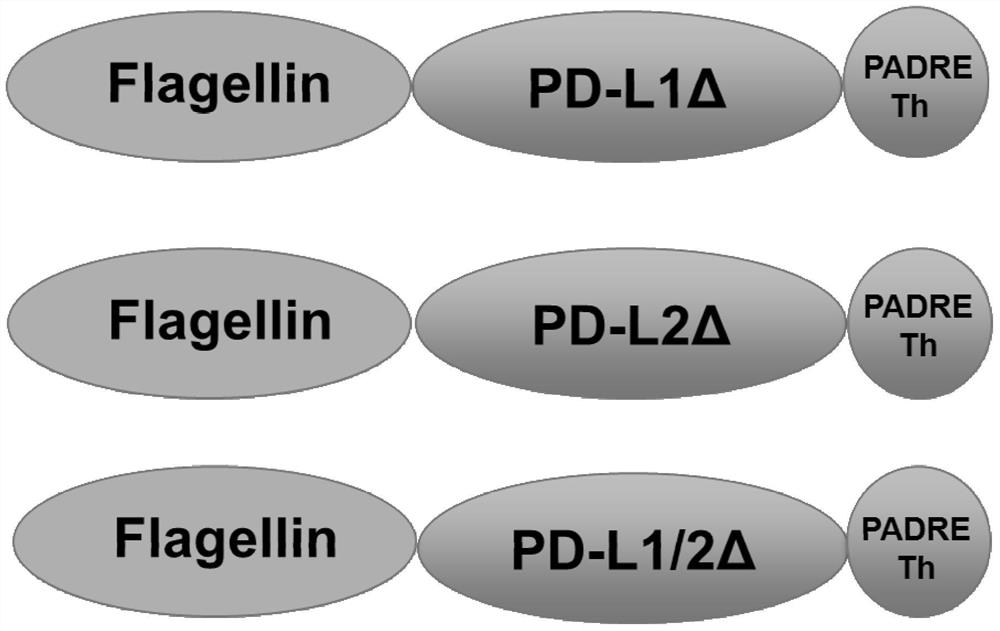
[0103] Synthetic fusion gene 1: contains the complete Flagellin sequence, part of the human PD-L1 sequence or PD-L2 sequence (Accession number GenBank: AF177937.1), and the complete helper T cell PADRE epitope sequence and flanking cloning site sequence ( fusion gene-structure figure 1 shown) (synthesized by GENEWIZ, South Plainfield, NJ, USA). Synthetic fusion gene 2: contains part of human PD-L1 sequence or PD-L2 sequence (GenBank: AF177937.1) and complete helper T cell PADRE epitope sequence. These synthetic genes were digested by restriction endonucleases NdeI and XhoI (purchased from BoehringerMannheim) and cloned into the pET21a(+) expression vector (purchased from Novagen), identified by enzyme digestion and sequencing, and connected correctly without mutations. .
Embodiment 3
[0104] Example 3 Preparation and Purification of a Fusion Protein Containing Human PD-L1
[0105] In order to prepare and purify recombinant proteins, target recombinant plasmids were electrotransfected into Escherichia coli BL21(DE3) (Novagen) competent cells, after which Escherichia coli BL21(DE3) were inoculated on LB agar culture plates (containing 50 μg / ml ampicillin) Carry out expansion culture. The method of recombinant protein expression described below is one of a series of experiments under different experimental conditions.
[0106] In order to express the recombinant protein, 4YT medium containing ampicillin (containing 32g Bacto tryptone, 20g yeast extract and 5g NaCl / L, pH 7.2) was incubated with one clone, the incubation conditions were: shaking culture at 180rpm and 37°C 24h. Then IPTG (purchased from Sigma) was added to a final concentration of 1 mM, and culture was continued for 4-5 hours to express the recombinant protein. Finally, the cells were collecte...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com