Preparation method of ferroferric oxide/ titanium dioxide magnetic biological activity coating
A technology of ferroferric oxide and titanium dioxide, applied in coatings, surface reaction electrolytic coatings, electrolytic coatings, etc., can solve problems such as implant failure, insufficient biological activity, and weak tissue bonding, so as to promote new bone formation, Good biological activity, promote integration effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
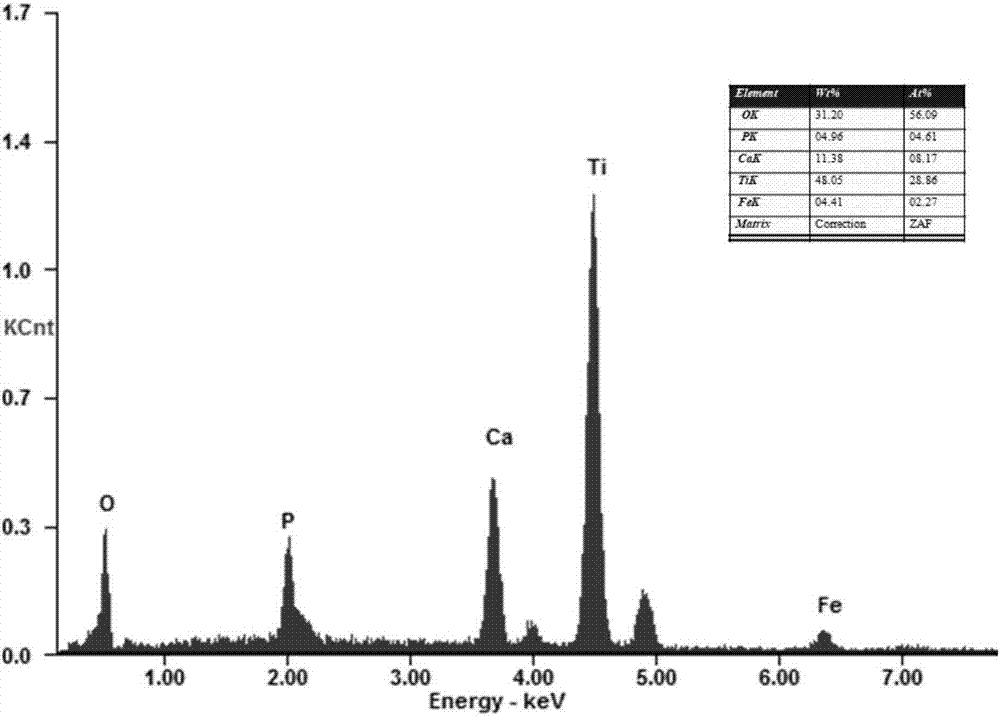
Image
Examples
preparation example Construction
[0027] A preparation method for ferroferric oxide / titanium dioxide magnetic bioactive coating, comprising the following steps:
[0028] Step 1: using solvothermal method, using ethylene glycol as solvent, 1,6-hexamethylenediamine, ferric chloride hexahydrate, and anhydrous sodium acetate as solutes, wherein the concentration of 1,6-hexamethylenediamine is 0.2–5.0 mol / L, the concentration of ferric chloride hexahydrate is 0.05-2.0mol / L, the concentration of anhydrous sodium acetate is 0.01-0.5mol / L, the reaction is carried out in a high temperature and high pressure reactor, the reaction temperature is 180-220°C, the time 2-24h, after the reaction is completed, water-soluble aminated ferric oxide nanoparticles with a particle size of 20-300nm are obtained;
[0029] Step 2: Using micro-arc oxidation method, water is used as solvent, and water-soluble aminated ferric oxide nanoparticles, calcium salt, phosphorus salt and weak acid are used as solute to form an electrolyte, and wa...
Embodiment 1
[0032] Step 1: Prepare 180mL of a solution with 1,6-hexanediamine, ferric chloride hexahydrate, anhydrous sodium acetate as the solute and ethylene glycol as the solvent, wherein the concentration of 1,6-hexanediamine is 1.4mol / L, the concentration of ferric chloride hexahydrate is 0.1mol / L, and the concentration of anhydrous sodium acetate is 0.06mol / L, then transfer the above solution into a 250mL high-temperature and high-pressure reactor, and keep it warm for 6 hours at 198°C to prepare Water-soluble aminated ferric oxide nanoparticles with a size of 160nm were obtained.
[0033] The second step: in the composite solution of 0.2mol / L calcium acetate, 0.2mol / L β-sodium glycerophosphate and 0.167mol / L acetic acid, add 5.0g of the above-mentioned aminated ferric oxide nanometer Particles form an electrolyte, with titanium as the anode and stainless steel as the cathode, using a pulse power supply to conduct micro-arc plasma treatment on titanium under the conditions of volta...
Embodiment 2
[0035] Step 1: Prepare 180mL of a solution with 1,6-hexanediamine, ferric chloride hexahydrate, anhydrous sodium acetate as the solute and ethylene glycol as the solvent, wherein the concentration of 1,6-hexanediamine is 0.2mol / L 1,6-hexamethylenediamine, the concentration of ferric chloride hexahydrate is 2.0mol / L, and the concentration of anhydrous sodium acetate is 0.01mol / L. The water-soluble aminated ferric oxide nanoparticles with a size of 20 nm can be prepared by keeping the temperature for 2 hours.
[0036] Second step: in the composite solution of the calcium chloride by 0.5mol / L, the sodium phosphate of 0.5mol / L and 0.01mol / L oxalic acid, add the above-mentioned aminated ferric oxide nanoparticles of 0.2g, An electrolyte is formed, with titanium as the anode and stainless steel as the cathode, using a pulse power supply to conduct micro-arc plasma treatment on titanium under the conditions of voltage 550V, frequency 350Hz, duty cycle 5%, and distance between cathod...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com