Photosensitive targeted antineoplastic prodrug for responding to glutathione to kill tumor cells and preparation method and application thereof
A technology of glutathione and tumor cells, which is applied in the direction of anti-tumor drugs, pharmaceutical formulas, medical preparations containing active ingredients, etc., can solve the problems of anti-tumor clinical application limitations, low treatment efficiency, and large toxic and side effects. To achieve the effect of improving adverse pharmacokinetics
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] The synthesis of embodiment 1 prodrug (CM-2)
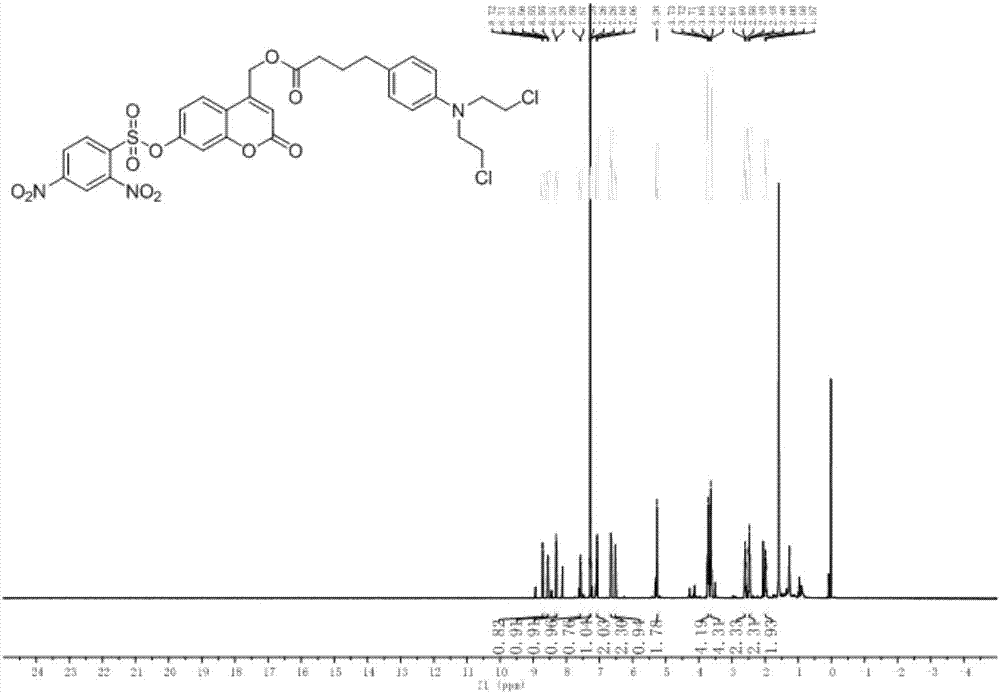
[0041] Put compound 2-3 (100 mg) into a three-necked flask containing 30 mL of DCM, after it is completely dissolved, add 1.5 equivalents of DMAP, 2.5 equivalents of DCC, and after activating for 10 minutes, dissolve 2.2 equivalents of chlorambucil in a 5 mL DCM injection bottle , reacted overnight. Add 50mL DCM to the bottle, wash with water (3×30mL), wash with saturated sodium chloride (2×30mL), dry over anhydrous sodium sulfate, filter, and remove the organic solvent by rotary evaporation to obtain a crude product, which is separated by thin-layer chromatography to obtain The product is pure product CM-2, the developing solvent used is DCM(D) / MeOH(M)=15:1, and the yield is 83%. H NMR spectrum see figure 1 .
[0042] 1 H NMR (500MHz, CDCl 3 )δ7.85(d,J=8.1Hz,3H),7.42(dd,J=16.4,8.4Hz,6H),7.08(d,J=8.7Hz,4H),6.97–6.88(m,4H), 6.68–6.60(m,4H),6.33(s,2H),5.25(d,J=1.2Hz,4H),5.16(d,J=3.6Hz,4H),3.71(t,J=6.9Hz,8H ), 3.63(dd, ...
Embodiment 3
[0045] Synthesis of embodiment 3 reference compound K
[0046] Compound 3 (100 mg) was put into a three-neck flask containing 30 mL of DCM. After it was completely dissolved, 0.2 equivalents of DMAP and 1.2 equivalents of DCC were added for activation for 30 minutes. 1.2 equivalents of chlorambucil was dissolved in 10 mL of DCM and injected into the bottle, and the reaction 24 hours. Add 50mL DCM to the bottle, wash with water (3×30mL), wash with saturated sodium chloride (2×30mL), dry over anhydrous sodium sulfate, filter, and rotary evaporate to remove the organic solvent to obtain a crude product, which is separated by thin-layer chromatography to obtain the product For pure product K, the developing solvent used is DCM(D) / MeOH(M)=15:1-3, and the yield is 51%.
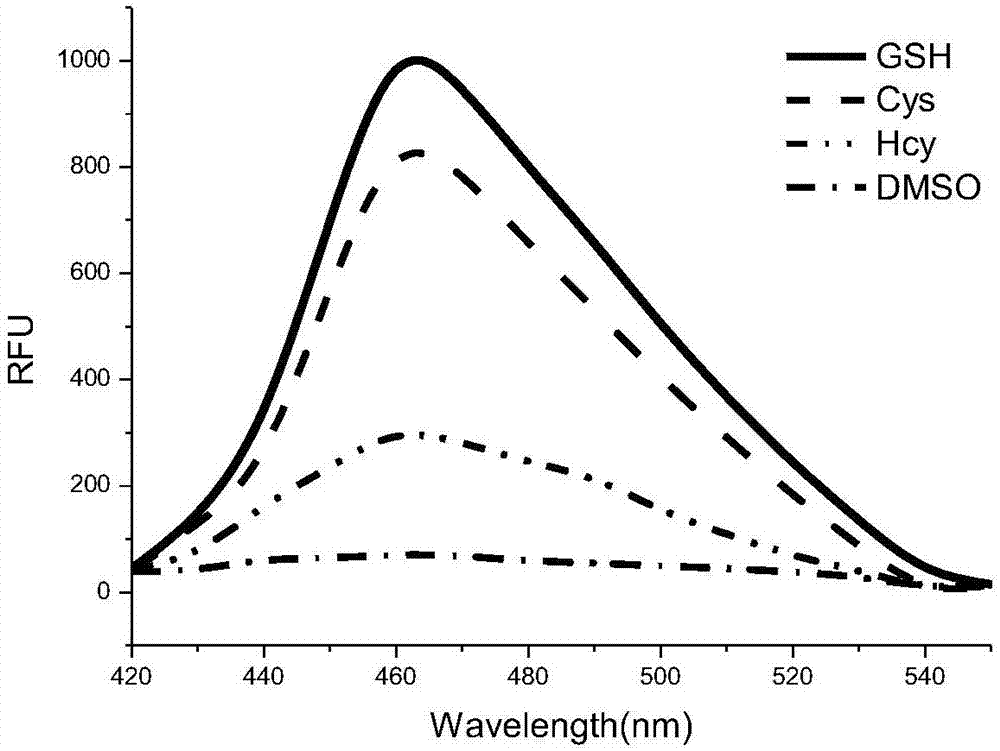
[0047] Example 4 The prodrug (CM-2) prepared in Example 1 was detected by the fluorescence spectrum of adding 0 mM and 100 μM glutathione aqueous solution concentration under the condition of pH 7.4.
[0048] Add gl...
Embodiment 12
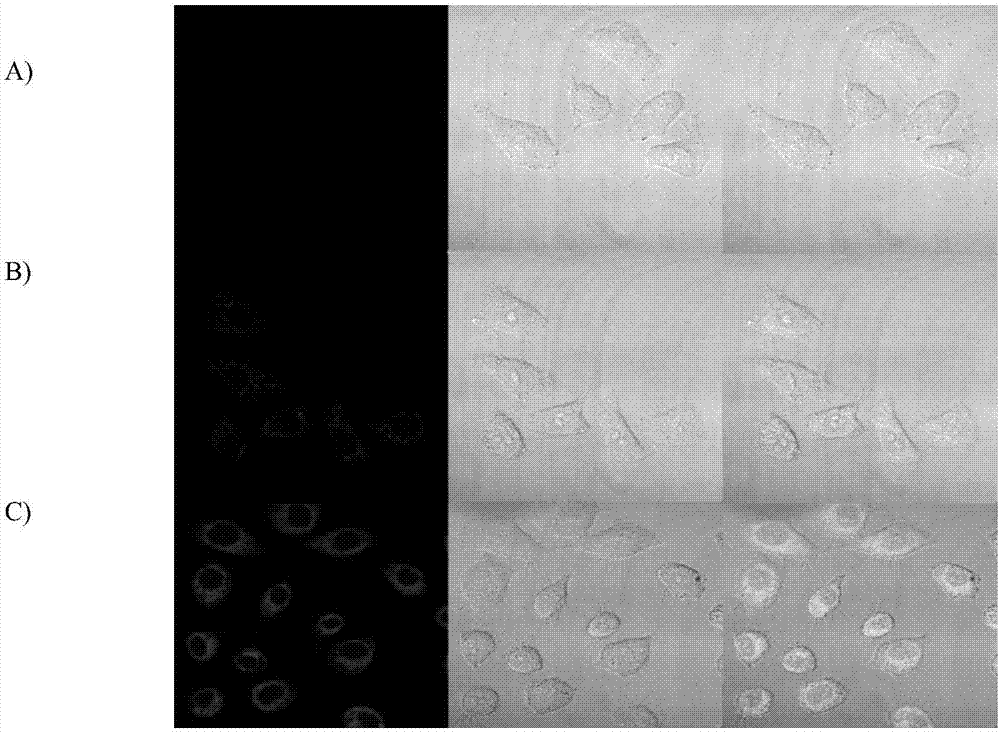
[0063] Example 12 Effect diagram of confocal fluorescence imaging of prodrug (CM-2) in cervical cancer cells (HeLa).
[0064] Since the coumarin released after the action of glutathione and light is a strong fluorescent dye, we tried to observe the distribution of the drug in the tumor cells through a confocal microscope. The experimental results are as follows: Figure 9 shown. Among them, Figure A is the untreated control group, Figure B is the confocal cell imaging image after drug addition and incubation for 12 hours, and Figure C is the glutathione treatment for 30 minutes after drug addition and incubation for 12 hours. It can be seen from the figure that the compound can be taken up by cells, and whether chlorambucil is released can be judged by the cell morphology at this time.
[0065] Experiments have shown that when the concentration of glutathione increases, we can see that the fluorescent signal in the cells also becomes stronger. Explain that our substance resp...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com