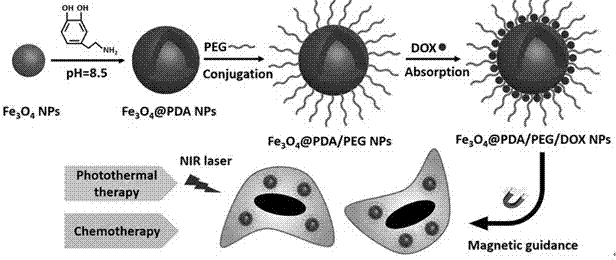
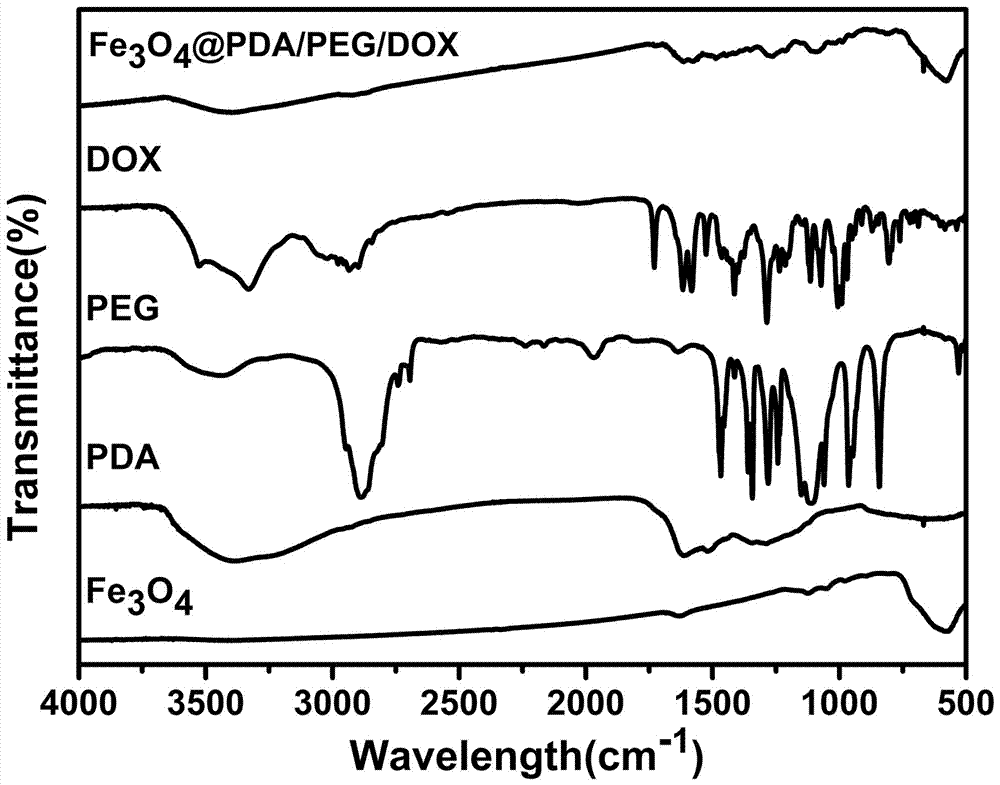
Preparation method of pegylated polydopamine-coated drug-loaded magnetic nanoparticles
A technology of magnetic nanoparticles and encapsulation of polydopamine, which is used in pharmaceutical formulations, medical preparations containing active ingredients, anti-tumor drugs, etc., to improve the therapeutic effect, solve the effect of targeting and synergy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Example 1 Preparation of PEGylated Drug-loaded Magnetic Nanoparticles Encapsulating Polydopamine
[0029] (1) Fe 3 o 4 Preparation of @PDA magnetic composite material: Dissolve 0.556g of ferrous sulfate heptahydrate and 1.04g of ferric chloride hexahydrate in 5mL of deionized water, add 0.17mL of concentrated hydrochloric acid, and add the mixed solution dropwise to 50mL of 1.5M hydroxide Stir vigorously in the sodium solution at 80°C under nitrogen protection for half an hour, cool to room temperature naturally, and wash the magnetic separation with deionized water for 3 times. Fe 3 o 4 Nanoparticles were dispersed in pH=8.5, 10mM Tris buffer solution, mechanically stirred at room temperature for 12 hours, magnetically separated, and washed three times with deionized water.
[0030] (2) PEGylated Fe 3 o 4 Preparation of @PDA: Add 100mg thiol polyethylene glycol to 40mL 1mg / mLFe 3 o 4 @PDA nanoparticle suspension, after stirring for 5 minutes, add 0.2mL ammonia w...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com