Method for separating and determining impurities in vitamin E and preparation thereof with HPLC (High Performance Liquid Chromatography) method
A vitamin and impurity technology, applied in drug analysis and detection, in the field of medicine, can solve the problems of cumbersome operation, poor reproducibility, harsh experimental conditions of gas chromatography, etc., and achieve the effect of high detection sensitivity and quality controllability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Instrument: Waters high performance liquid chromatography;
[0037] Column: Wondasil C 18 Chromatographic column (250×4.6mm, 5μm);
[0038] Mobile Phase: Mobile Phase A: Acetonitrile-Ethanol-Water (35:10:55)
[0039] Mobile phase B: acetonitrile-ethanol-methanol (45:15:40);
[0040] Gradient elution program:
[0041] time (minutes)
Mobile phase A (%)
Mobile phase B (%)
0
20
80
110
20
80
112
10
90
140
10
90
142
20
80
150
20
80
[0042] Detection wavelength: 272nm;
[0043] Column temperature: 30°C
[0044] Flow rate: 1.0mL / min;
[0045] Preparation of dl-α-tocopherol acetate peak identification reference substance solution: Take about 7.5 mg of dl-α-tocopherol acetate peak identification reference substance, dissolve it in 5 ml of absolute ethanol, shake well, and obtain.
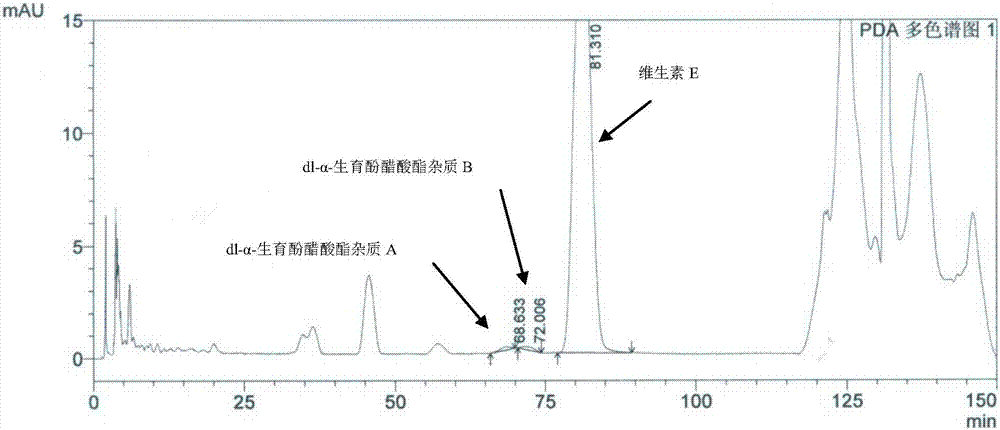
[0046]Preparation of vitamin E test solution: Take an appropriate amount of vitamin E raw material,...
Embodiment 2
[0054] Instrument: Waters high performance liquid chromatography;
[0055] Column: Wondasil C 18 Chromatographic column (250×4.6mm, 5μm);
[0056] Mobile phase: mobile phase A: acetonitrile-ethanol-water (40:10:50)
[0057] Mobile phase B: acetonitrile-ethanol-methanol (45:20:25);
[0058] Gradient elution program:
[0059] time (minutes)
Mobile phase A (%)
Mobile phase B (%)
0
25
75
110
25
75
112
0
100
140
0
100
142
25
75
150
25
75
[0060] Detection wavelength: 292nm;
[0061] Column temperature: 30°C
[0062] Flow rate: 1.0mL / min;
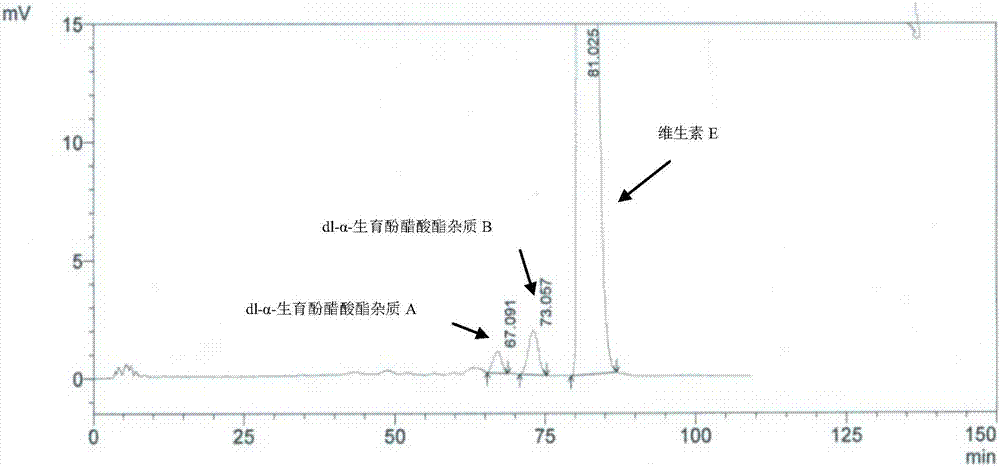
[0063] Solution preparation method and assay method are carried out according to " containing vitamin E preparation test sample solution " in embodiment 1, and the results are shown in Figure 5 .
Embodiment 3
[0065] Instrument: Waters high performance liquid chromatography;
[0066] Column: Wondasil C 18 Chromatographic column (250×4.6mm, 5μm);
[0067] Mobile Phase: Mobile Phase A: Acetonitrile-Ethanol-Water (40:15:45)
[0068] Mobile phase B: acetonitrile-ethanol-methanol (35:25:40);
[0069] Gradient elution program:
[0070] time (minutes)
Mobile phase A (%)
Mobile phase B (%)
0
15
85
110
15
85
112
5
95
140
5
95
142
15
85
150
15
85
[0071] Detection wavelength: 270nm;
[0072] Column temperature: 40°C
[0073] Flow rate: 1.1mL / min;
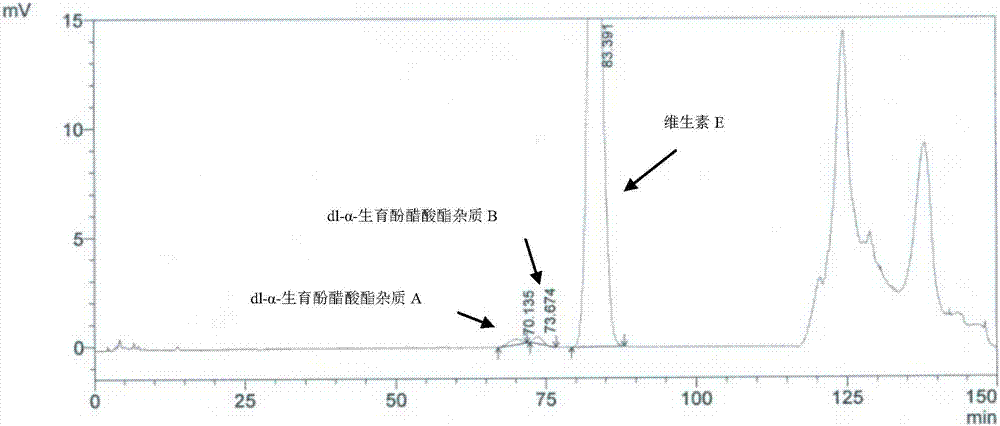
[0074] Solution preparation method and assay method are carried out according to " containing vitamin E preparation test sample solution " in embodiment 1, and the results are shown in Figure 6 .
PUM
| Property | Measurement | Unit |
|---|---|---|
| Granularity | aaaaa | aaaaa |
| The inside diameter of | aaaaa | aaaaa |
| Length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com