Construction method and application of genetic engineering strain for producing (r,r)-2,3-butanediol
The technology of a genetically engineered strain and a construction method is applied in the construction of a genetically engineered strain for producing high optical purity-2,3-butanediol, and the application field in the production of high optical purity-2,3-butanediol, which can solve the problem of The optical purity does not meet the requirements, the production capacity is insufficient, and the yield of butanediol is low.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
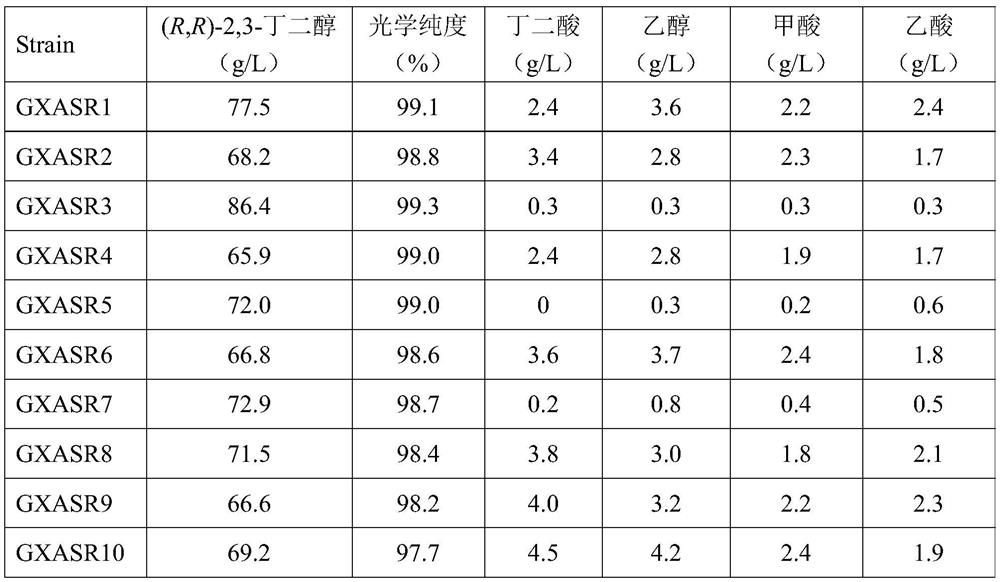
[0032] Construction of the genetically engineered strain GXASR1 for the production of (R,R)-2,3-butanediol:
[0033] The genome sequence of Paenibacillus polymyxa DSM 365 was analyzed, and the (R,R)-2,3-butanediol dehydrogenase gene bdh of the strain was obtained, and it was combined with the α-acetolactate synthase gene budB, The nucleotide sequence of the α-acetolactate decarboxylase gene budA was codon-optimized, and the nucleotide sequence TAAGGAGGATATACA containing the ribosome binding site was added in front of each gene, and then the gene cluster budB-budA- The length of bdh's nucleotide sequence is 3574 bases, and the nucleotide sequence is as described in SEQ ID NO.1. The gene cluster budB-budA-bdh was inserted behind the promoter of the plasmid pTrc99A by double enzyme digestion and ligation to obtain the polycistronic recombinant plasmid pTrc99A-budB-budA-bdh, and then the recombinant plasmid pTrc99A-budB-budA- The bdh was introduced into the host strain E.coli MG1...
Embodiment 2
[0035] The construction of the genetically engineered strain GXASR2 producing (R,R)-2,3-butanediol:
[0036] The genome sequence of Paenibacillus polymyxa DSM 365 was analyzed, and the nucleotide sequences of the α-acetolactate synthase gene alsS and α-acetolactate decarboxylase gene alsD of the strain were obtained. Codon optimization was performed on the nucleotide sequences of alsS, alsD and the (R,R)-2,3-butanediol dehydrogenase gene bdh from Paenibacillus polymyxa, and a ribosome binding site was added in front of each gene The nucleotide sequence TAAGGAGGATATACA, and then use artificial synthesis method to obtain the gene cluster alsS-alsD-bdh, its nucleotide sequence length is 3562 bases, the nucleotide sequence is as described in SEQ ID NO.2. Then insert the gene cluster alsS-alsD-bdh behind the promoter of the plasmid pTrc99A to obtain the polycistronic recombinant plasmid pTrc99A-alsS-alsD-bdh, which is introduced into the host strain E.coli MG1655 to obtain the (R,R...
Embodiment 3
[0038] Construction of the genetically engineered strain GXASR3 for the production of (R,R)-2,3-butanediol:
[0039] Through analysis, it was found that the main by-products of the fermentation of engineering strains GXASR1 and GXASR2 were meso-2,3-butanediol, succinic acid, formic acid, ethanol and acetic acid, and the key genes of the synthesis pathway were dar, frdABCD, pflB, adhE and pta . Using the principle that the Red recombination system derived from Escherichia coli λ phage can efficiently mediate homologous recombination events in bacteria, first replace the above target gene with an antibiotic resistance gene with FRT sites on both sides, and then induce exogenous temperature The sensitive plasmid expresses FLP recombinase to delete the antibiotic resistance gene to achieve the purpose of knocking out the target gene. The specific steps are as follows:
[0040] Transform the pKD46 plasmid into host cells to prepare electroporation-competent cells; use primers to c...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size (mesh) | aaaaa | aaaaa |
| particle size (mesh) | aaaaa | aaaaa |
| particle size (mesh) | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com