A kind of tanshinone skeleton splicing bisindole or bispyrrole compound and its preparation method and application
A technology of tanshinone and bisindole, applied in the field of tanshinone skeleton splicing bisindole or bispyrrole compounds and its preparation, to achieve the effect of cheap raw material synthesis, good air stability and good compatibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
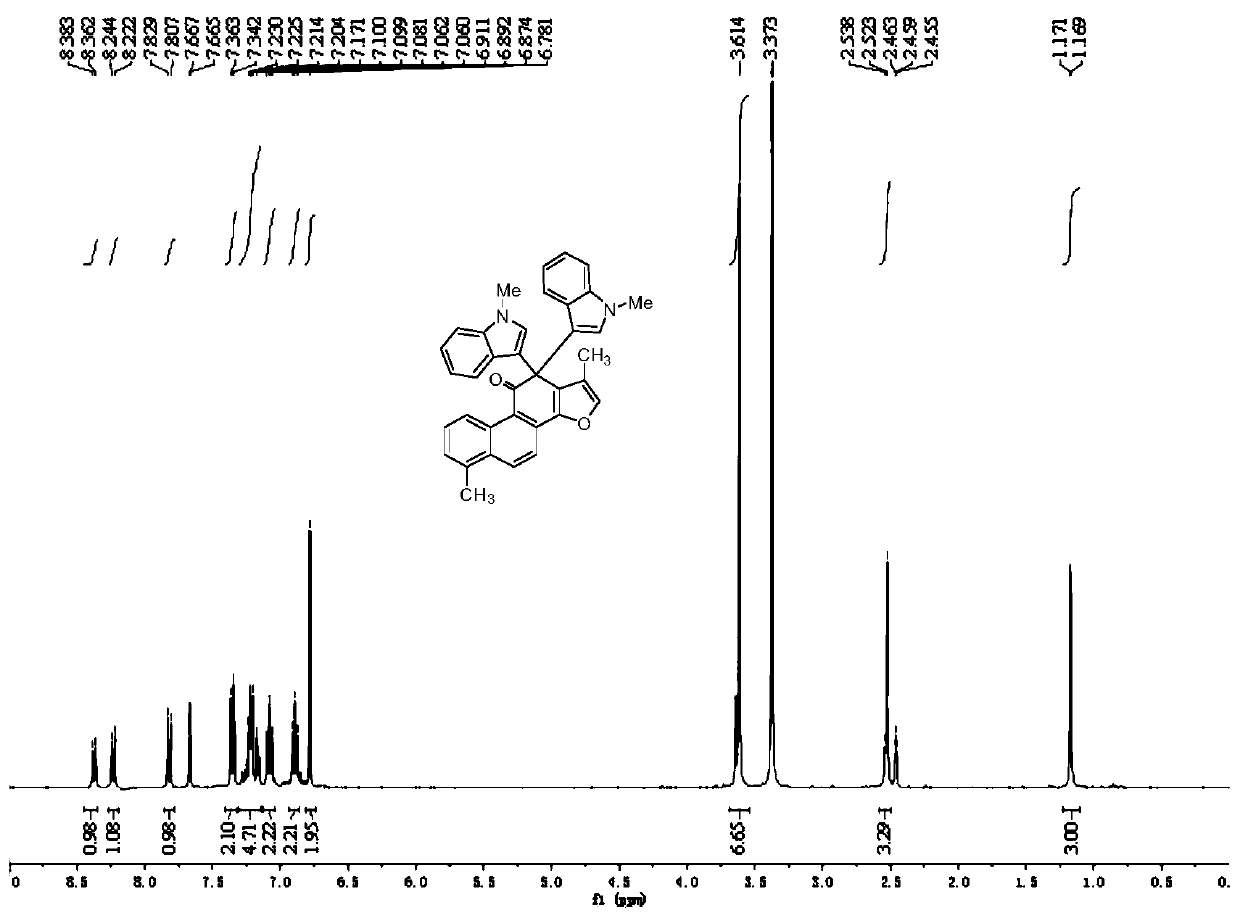
[0025] The preparation method of compound 3b-3r is the same as that of compound 3a, and the feeding ratio is the same as that of compound 3a. Compound 3b-3r can be obtained. The reaction yield is shown in Table 1 and Table 2, but it should be emphasized that the compounds of the present invention are not limited to Table 1 and Table 2. 2 represents the content.
[0026] Table 1 is the chemical structure of a kind of tanshinone skeleton splicing bisindole or bispyrrole compound
[0027]
[0028] Table 2 is the chemical structure of a kind of tanshinone skeleton splicing bisindole or bispyrrole compound
[0029]
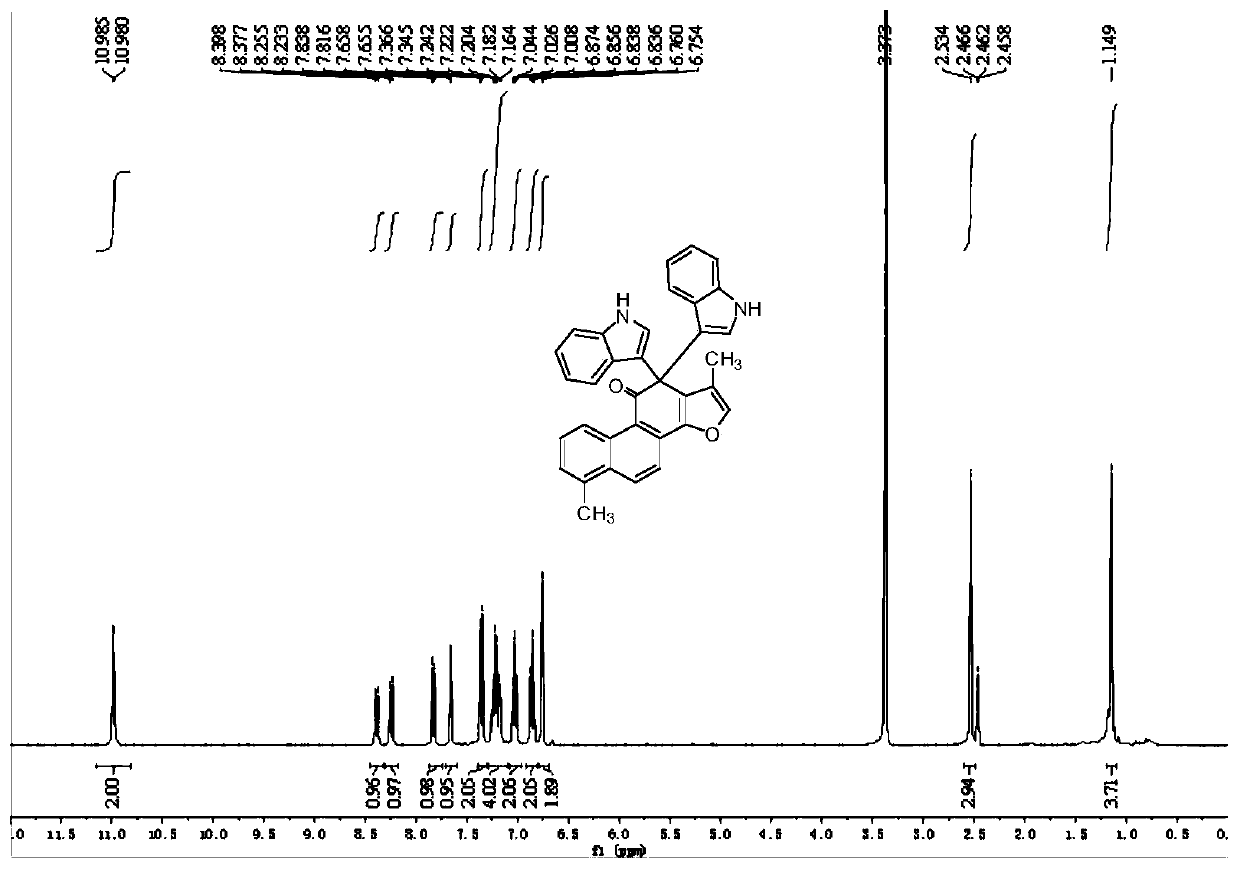
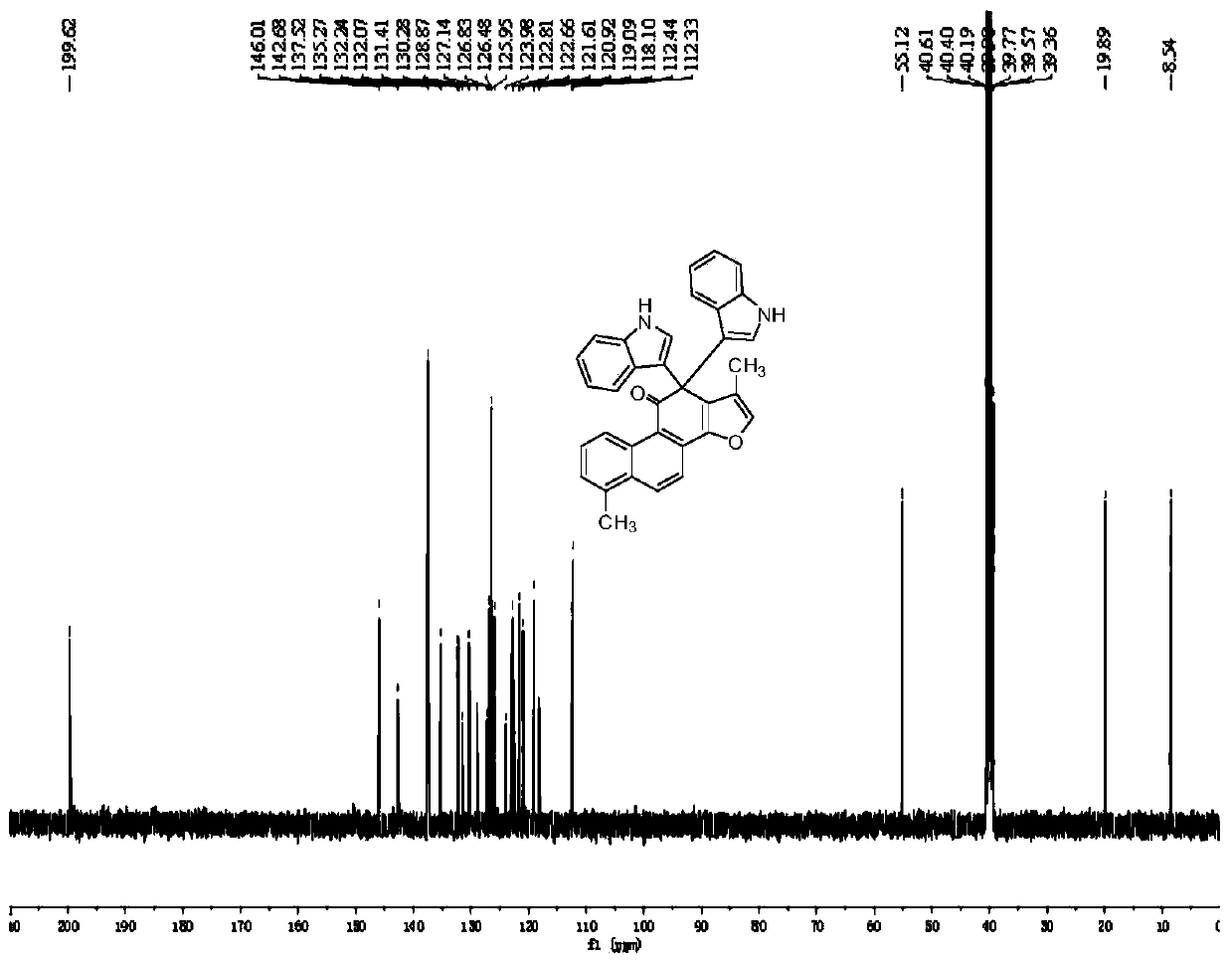
[0030] Compound 3b prepared in this example: yellow solid, melting point: 222.9-223.4°C, yield 69%; NMR and high-resolution mass spectrometry results are as follows: 1 H NMR (DMSO-d 6 ,400MHz)δ:1.17(s,3H),2.52(s,3H),3.61(s,6H),6.78(s,2H),6.87-6.91(m,2H),7.06-7.10(m,2H) ,7.15-7.28(m,5H),7.35(d,J=8.4Hz,2H),7.81(d,J=8.8Hz 1H),8.23(d,J=8.8Hz,1H),8.37(d,J =8.4Hz,1...
Embodiment 1
[0048] Pharmacological Example 1: Cytotoxicity of compounds 3a, 3b, 3d, 3e, 3f, 3g, 3i, 3j, 3k, 3l, 3m, 3n, 3p and 3r on PC-3 cells
[0049] PC-3 (human prostate cancer) cells were cultured with RPMI-1640 medium containing 10% fetal bovine serum, 100 U / mL penicillin and 100 U / mL streptomycin. Cells were added to 96 wells at a concentration of 5000 cells per well at 37°C with 5% CO 2 Incubate for 24 hours in a humidified incubator.
[0050] Cell viability was determined by the modified MTT method. After the cells were incubated for 24 hours, the newly prepared compounds 3a, 3b, 3d, 3e, 3f, 3g, 3i, 3j, 3k, 3l, 3m, 3n, 3p and 3r were added to the dimethyl sulfoxide solution at a concentration of Gradients were added to each well so that the final concentrations of the compounds in the wells were 5 μmol / L, 10 μmol / L, 20 μmol / L, 40 μmol / L and 80 μmol / L, respectively. After 48 hours, add 10 μL of MTT (5 mg / mL) in phosphate buffered saline to each well, continue to incubate at 37°...
Embodiment 2
[0052] Pharmacological Example 2: Cytotoxicity of compounds 3a, 3b, 3d, 3e, 3f, 3g, 3i, 3k, 3l, 3m, 3n and 3r on A549 cells
[0053] A549 (human non-small cell lung cancer) was cultured with DMEM medium containing 10% fetal bovine serum, 100 U / mL penicillin and 100 U / mL streptomycin. Cells were added to 96 wells at a concentration of 4000 cells per well at 37°C with 5% CO 2 Incubate for 24 hours in a humidified incubator.
[0054] Cell viability was determined by the modified MTT method. The specific method is as in Pharmacological Example 1. IC of compound 3a on A549 tumor cells 50 It was 23.7μmol / L; the IC of compound 3b on A549 tumor cells 50 It was 77.6μmol / L; the IC of compound 3d on A549 tumor cells 50 It was 69.9μmol / L; the IC of compound 3e on A549 tumor cells 50 It was 45.6μmol / L; the IC of compound 3f on A549 tumor cells 50 It was 39.1 μmol / L; the IC of compound 3g on A549 tumor cells 50 It was 43.6μmol / L; the IC of compound 3i on A549 tumor cells 50 It was ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com