Method for synthesizing multi-substituted benzene by one reactor method
A multi-substitution and benzo technology, applied in organic chemistry and other fields, can solve the problems of cumbersome transformation steps and achieve the effects of simple operation, good water resistance and air stability, and cheap and easy synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
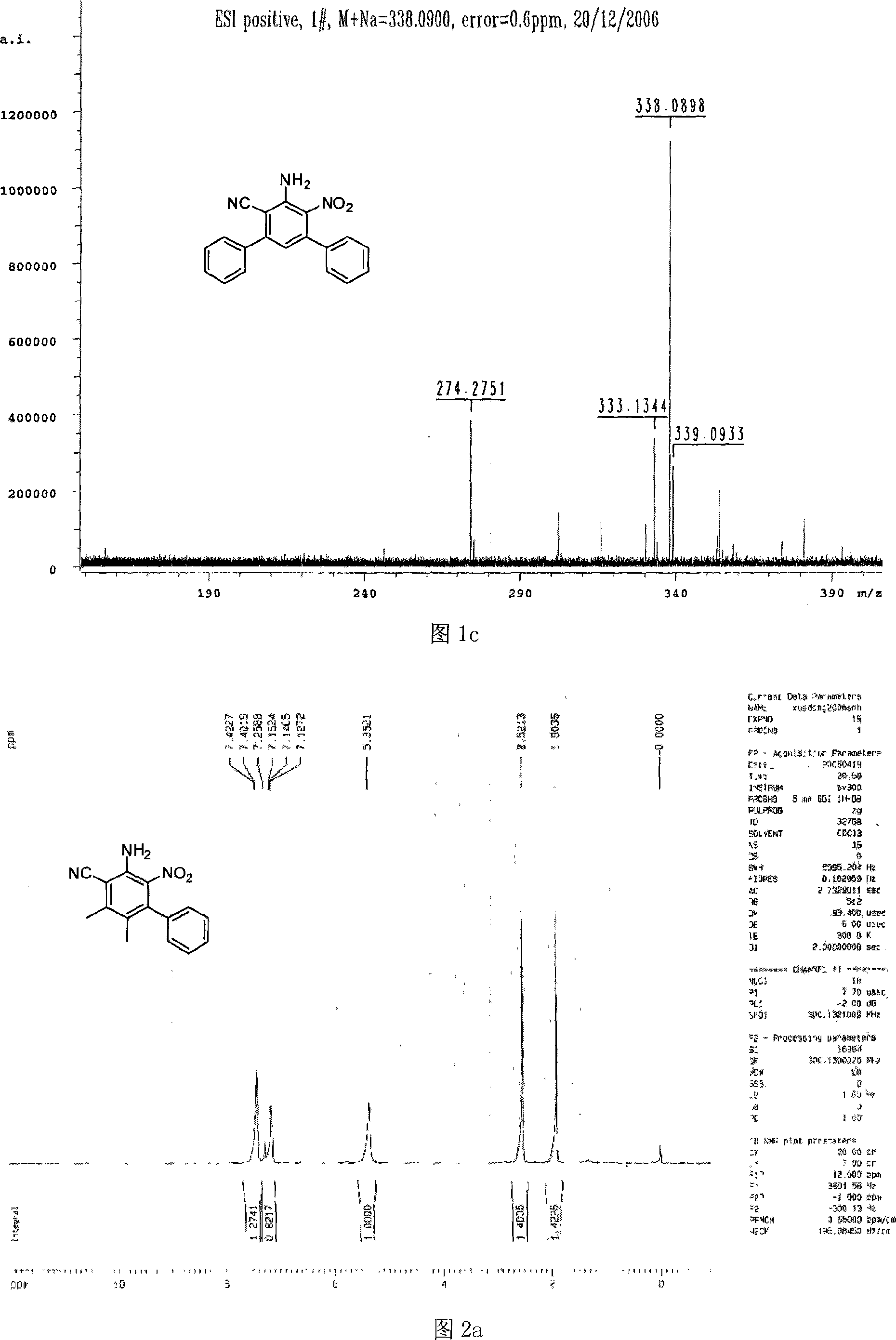
[0025] Compound 1: Add 15 ml of acetonitrile, raw material 1 (0.168 g, 1 mmol) and raw material 2 (0.149 g, 1 mmol) into a dry flask, stir well to completely dissolve the substrate, heat up to 60°C, add Sodium ethoxide (68 mg, 1 mmol, 100%), under vigorous stirring to completely dissolve the sodium ethoxide. The reaction mixture was kept warm (100°C) until the reaction of starting materials was complete (2 hours). After cooling to room temperature, the solvent was distilled off and separated by column chromatography (petroleum ether: ethyl acetate = 6:1) to obtain a pale yellow solid.
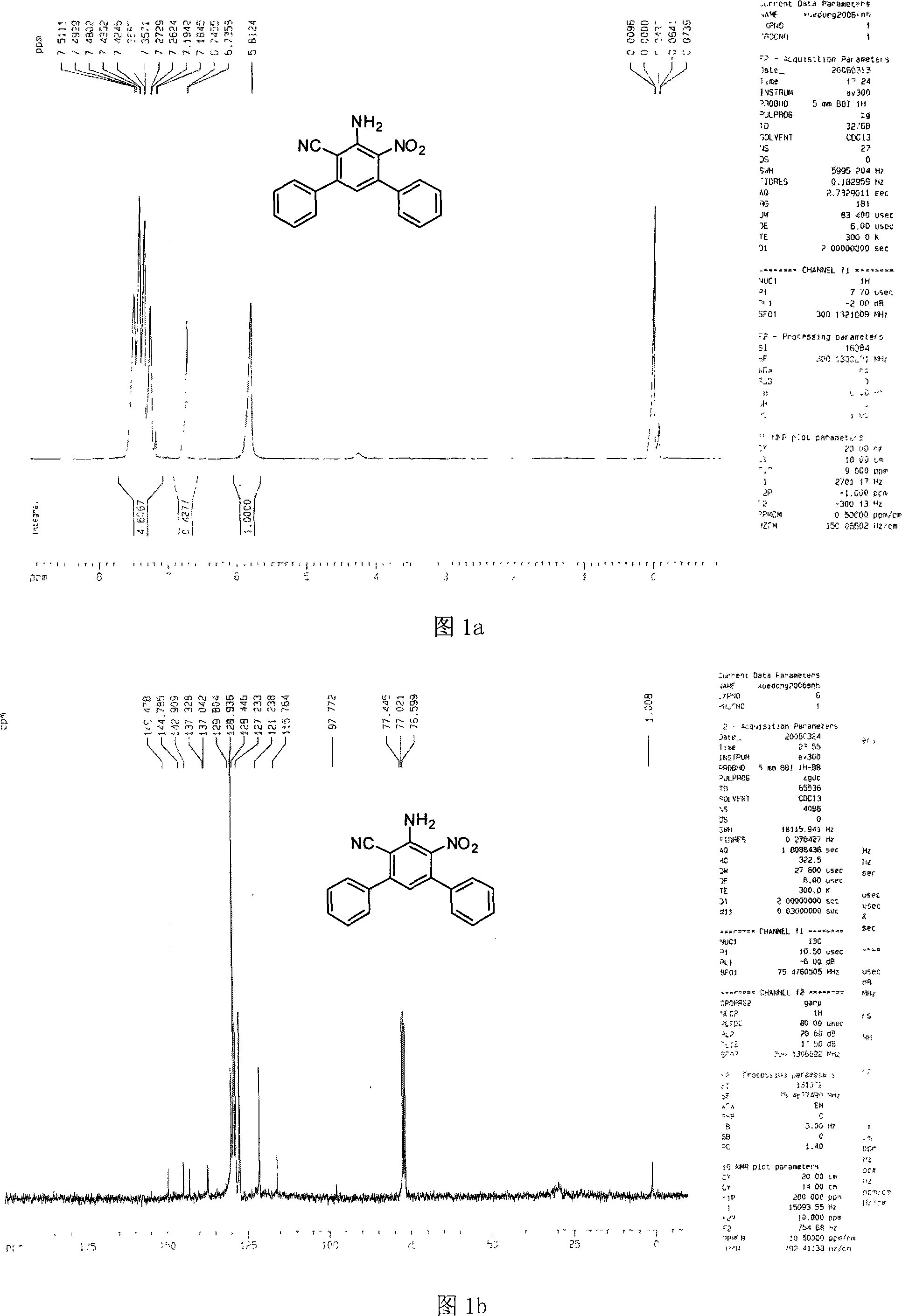
[0026] The physical and chemical properties of compound 1 prepared in this example are as follows after testing: yield: 76%, melting point: 204.2-205.8°C;
[0027] Referring to Fig. 1a, the H of the compound 1 of the present embodiment is tested with a nuclear magnetic resonance instrument and the results are as follows: 1 H NMR (300MHz, CDCl 3 )δ (ppm) 5.81 (s, 2H), 6.74-7.51 (m, 11H);
[...
Embodiment 2
[0092] Compound 1: Add 15 ml of ethanol, raw material 1 (0.168 g, 1 mmol) and raw material 2 (0.149 g, 1 mmol) into a dry flask, stir well to completely dissolve the substrate, heat up to 60°C, add Sodium ethoxide (68 mg, 1 mmol, 100%), under vigorous stirring to completely dissolve the sodium ethoxide. The reaction mixture was kept at 60°C until the reaction of starting materials was completed (5 hours). After cooling to room temperature, the solvent was distilled off and separated by column chromatography (petroleum ether: ethyl acetate = 6:1) to obtain a pale yellow solid. Yield: 54%.
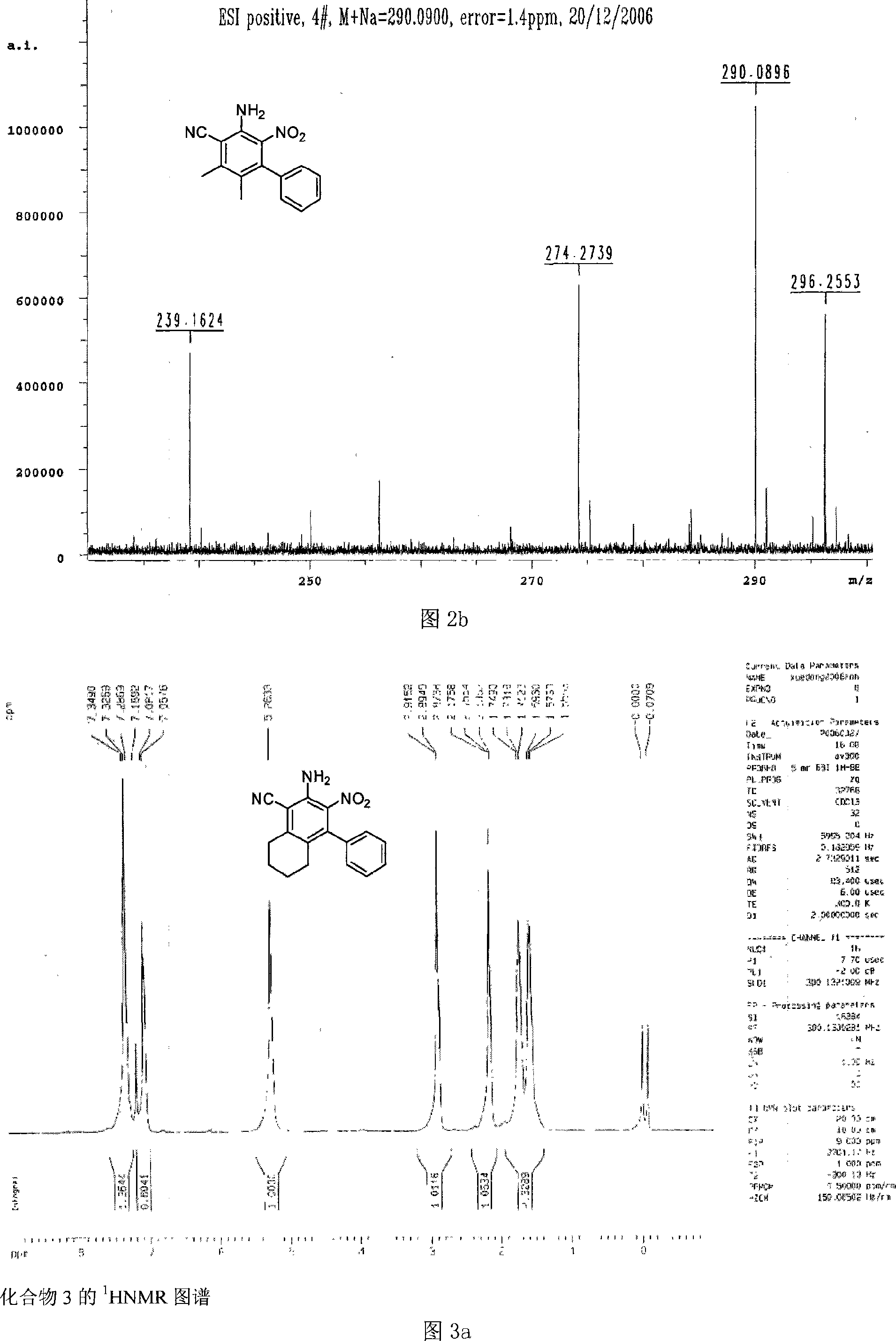
[0093] Substitute raw material 1 with raw material 2~14, its charging ratio is identical with compound 1, can obtain respectively compound 2 (productive rate 53%), compound 3 (productive rate 52%), compound 4 (productive rate 56%), compound 5 ( Yield 55%), compound 6 (yield 58%), compound 7 (yield 56%), compound 8 (yield 57%), compound 9 (yield 49%), compound 10 (yield 51%) , compound 11 ...
Embodiment 3
[0095] Compound 1: Add 15 ml of chloroform, raw material 1 (0.168 g, 1 mmol) and raw material 2 (0.149 g, 1 mmol) into a dry flask, stir well to completely dissolve the substrate, heat up to 60°C, add Sodium methoxide (54 mg, 1 mmol, 100%), the sodium methoxide was completely dissolved under vigorous stirring. The reaction mixture was kept warm (80°C) until the reaction of starting materials was complete (24 hours). After cooling to room temperature, the solvent was distilled off and separated by column chromatography (petroleum ether: ethyl acetate = 6:1) to obtain a pale yellow solid. Yield: 37%.
[0096] Substitute raw material 1 with raw material 2~14, its charge ratio is identical with compound 1, can obtain compound 2 (productive rate 33%), compound 3 (productive rate 32%), compound 4 (productive rate 40%), compound 5 ( Yield 35%), compound 6 (yield 38%), compound 7 (yield 36%), compound 8 (yield 37%), compound 9 (yield 40%), compound 10 (yield 41%) , compound 11 (yie...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com