A kind of o-carboxymethyl-n,n-double chain long alkylated chitosan oligosaccharide and its preparation method and application
A technology of carboxymethyl chitooligosaccharides and chitooligosaccharides, which is applied in the field of O-carboxymethyl-N, N-double-chain long alkylated chitosan and its preparation and application, can solve the problem of large volume of nanovesicles , high molecular weight of chitosan, low yield and other problems, to achieve the effect of controllable permeability, easy degradation and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
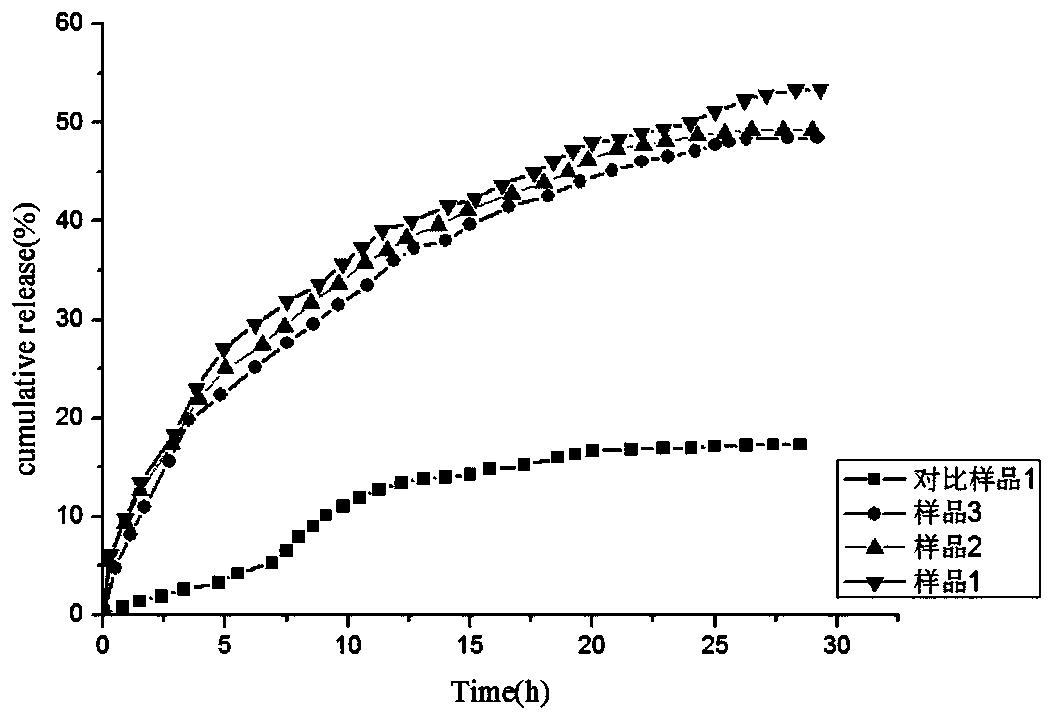
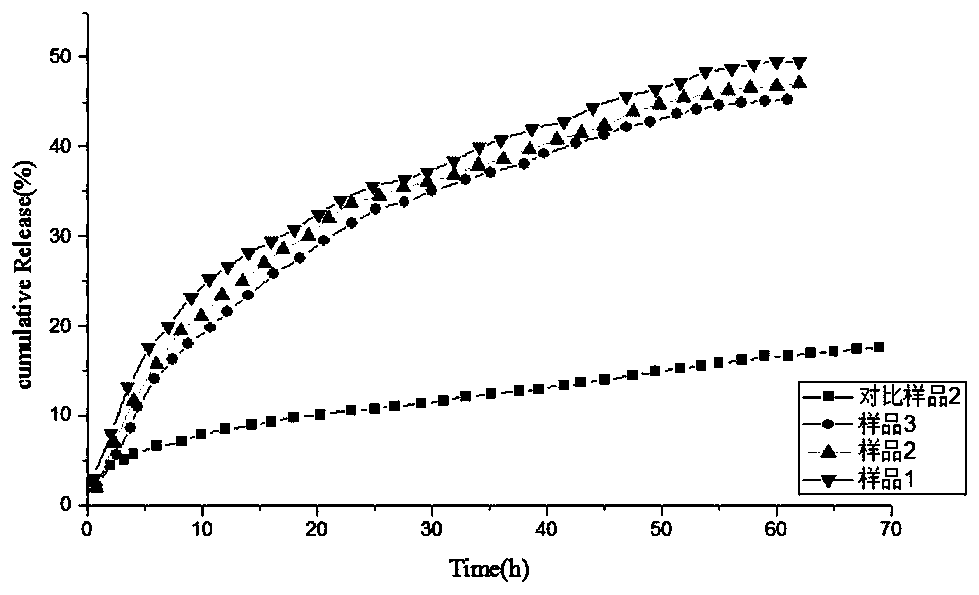
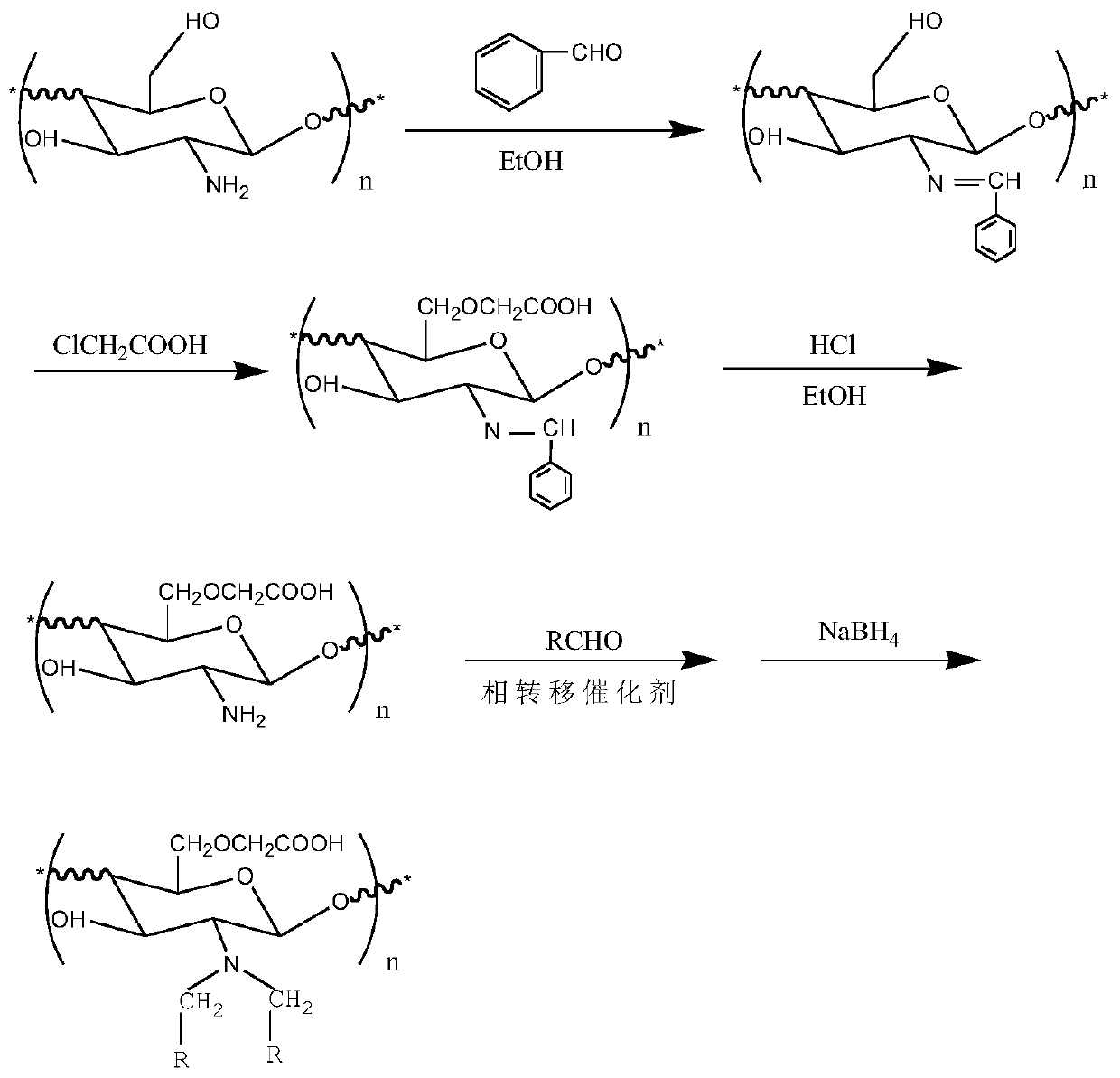
[0034] O-carboxymethyl-N, N-double-chain long alkylated chitosan can be prepared through the preparation route of the present invention. First, it not only has antibacterial properties and moisture retention, but also maintains the original good composition of chitosan. membrane properties, biocompatibility and biodegradability. Secondly, the chitosan oligosaccharide not only has a carboxymethyl group, but also has a long alkyl side chain, which makes it amphiphilic, thus providing a basis for the preparation of vesicles with excellent performance.
[0035] The application provides a method for protecting the amino group of a preferred chitosan oligosaccharide, the steps of which are: preparing the chitosan oligosaccharide into an aqueous solution of chitosan oligosaccharide, dissolving the small molecule aldehyde compound in an organic solvent one to prepare an aldehyde solution, and dissolving The aldehyde solution is added to the chitosan oligosaccharide mixed solution, and...
Embodiment 1
[0067] (1) Synthesis of N-benzylidene chitooligosaccharides
[0068] Dissolve 5g of chitosan oligosaccharide in 100mL of deionized water, stir at room temperature to fully dissolve, then add 10mL of absolute ethanol, weigh 6.58g of benzaldehyde, dissolve it in 10mL of absolute ethanol, add to the above solution, stir, and react at 30°C for 4h. 4 times the volume of ethanol was settled, washed twice with ethanol, filtered with suction, and dried in vacuum to obtain N-benzylidene chitosan oligosaccharide.
[0069] (2) Synthesis of O-carboxymethyl-N-benzylidene chitooligosaccharides
[0070] Weigh 4g of the dried product in (1), swell in 20mL of isopropanol at room temperature for 1h, then place in an ice-salt bath to cool for 2h, slowly add 30mL of 50% NaOH solution dropwise, stir evenly, and continue in the ice-salt bath After swelling for 2 hours, slowly add 5 mL of isopropanol solution containing 3.23 g of chloroacetic acid dropwise. Stir in a water bath at 50°C for more th...
Embodiment 2
[0078] (1) Synthesis of N-benzylidene chitooligosaccharides
[0079] Dissolve 5g of chitosan oligosaccharide in 100mL of deionized water, stir at room temperature to fully dissolve, then add 10mL of absolute ethanol, weigh 6.58g of benzaldehyde, dissolve it in 10mL of absolute ethanol, add to the above solution, stir, and react at 30°C for 4h. 4 times the volume of ethanol was settled, washed twice with ethanol, filtered with suction, and dried in vacuum to obtain N-benzylidene chitosan oligosaccharide.
[0080] (2) Synthesis of O-carboxymethyl-N-benzylidene chitooligosaccharides
[0081] Weigh 4g of the dried product in (1), swell in 20mL of isopropanol at room temperature for 1h, then place it in an ice-salt bath to cool for 1h, slowly add 30mL of 50% NaOH solution dropwise, stir evenly, and continue in the ice-salt bath After swelling for 2 hours, slowly add 5 mL of isopropanol solution containing 3.23 g of chloroacetic acid dropwise. Stir in a water bath at 50°C for more...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com