Recombinant protein of pulmonary prosurfactant protein-B (proSP-B) as well as preparation method and application of recombinant protein
A pulmonary surfactant, recombinant protein technology, applied in the fields of peptide/protein components, recombinant DNA technology, chemical instruments and methods, etc., can solve the problems of increasing the development and research of substances, difficult, and only occur, and achieve good broad-spectrum antibacterial. Activity, high safety, low cytotoxic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] This example is used to illustrate the preparation method of proSP-B recombinant protein provided by the present invention.
[0058] 1. Extraction of total RNA from rat lung tissue
[0059] (1) Total RNA was extracted from rat lung tissue using a total RNA extraction kit (purchased from GeneCopoeia, USA);
[0060] (2) Electrophoretic detection of RNA
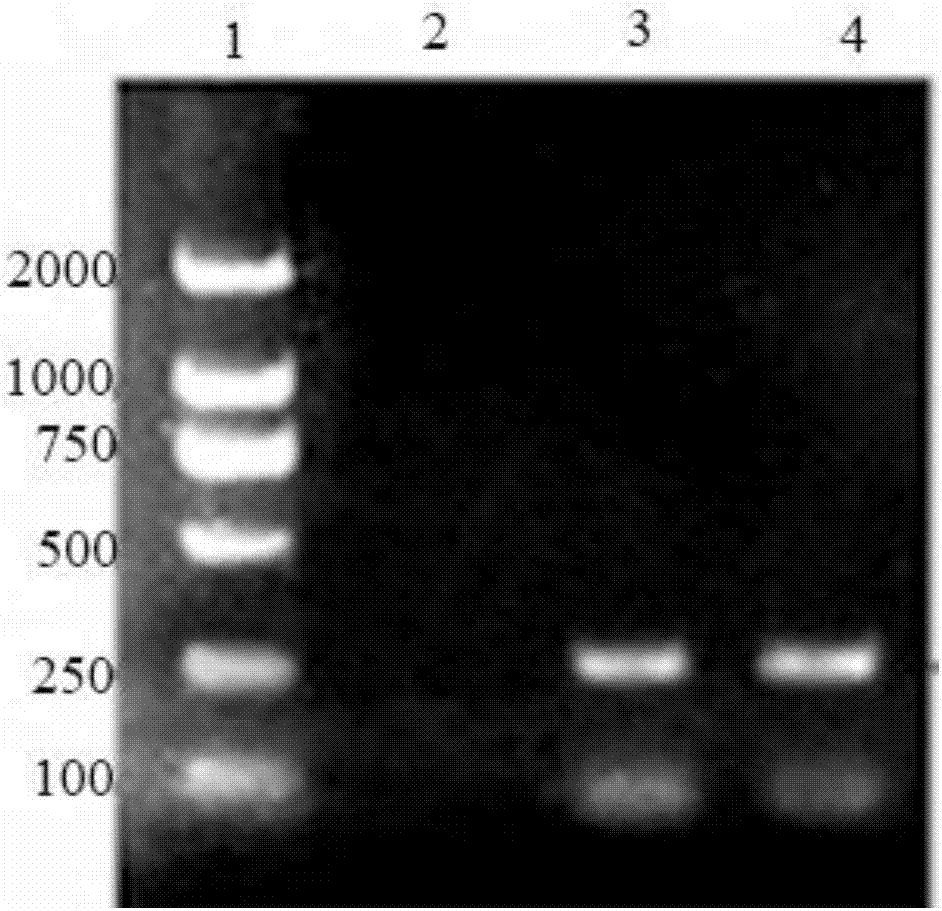
[0061] Use a newly configured gel electrophoresis solution for electrophoresis, and a gel imager to detect and analyze the quality of RNA.
[0062] 2. Acquisition and amplification of proSP-B gene cDNA
[0063] (1) Obtain cDNA fragments
[0064] Reverse transcription reaction system:
[0065] Total RNA 1 μg
[0066] Oli(dT) 18 1μL
[0067] Free RNase Water to 13μL
[0068] React at 65°C for 10 min, then store at 4°C.
[0069] reaction system:
[0070]
[0071] Reaction conditions: react at 42°C for 60 minutes, then react at 85°C for 5 minutes, and then store at 4°C. Finally, the reverse transcription produc...
Embodiment 2
[0124] This example is used to illustrate the cytotoxicity of proSP-B recombinant protein provided by the present invention
[0125] (1) CCL-149 cells were cultured in R / MINI1640 medium containing 10% FBS.
[0126] (2) Culture stable CCL-149 cells, digest the cells with trypsin, collect the cells and dilute them with medium to adjust the cell concentration, so that there are about 5000 cells in 100 μL, inoculate in the 1st to 10th wells of a 96-well plate 100 μL of CCL-149 cells, add 100 μL of culture medium in the 11th well, 37 ° C, 5% CO 2 Cultured in a cell culture incubator for 24 hours.
[0127] (3) Add 10 μL of 1000 μg / mL, 500 μg / mL, 250 μg / mL, 125 μg / mL, 62.5 μg / mL, 21.25 μg / mL, 15.63 μg / mL, 7.81 For μg / mL, 3.91 μg / mL purified protein obtained from step (7) in Example 1, add 10 μL of sterilized distilled water to the 10th and 11th wells.
[0128] (4) Place the 96-well plate at 37°C, 5% CO 2 Cultured in the cell culture incubator for 12h.
[0129] (5) Add 10 μL of C...
Embodiment 3
[0142] This embodiment is used to illustrate the antibacterial activity of the proSP-B recombinant protein provided by the present invention
[0143] 1. Activation of strains and preparation of bacterial suspension
[0144] (1) Cultivation of Staphylococcus aureus: activate Staphylococcus aureus on LB agar plate by streaking method, culture at 37°C for 12 hours at a constant temperature, after a single colony grows, pick a single colony and inoculate it in 10mL LB liquid medium medium, 37°C, 12h, 150r / min, cultivate, pipette gun to absorb the bacterial solution and sterilized double distilled water to make a bacterial suspension, adjust its concentration, and obtain 10 6 cfu bacterial suspension.
[0145] (2) Culture of Escherichia coli: activate Escherichia coli on LB agar plate by streaking method, culture at constant temperature for 12 hours at 37°C, after a single colony grows, pick a single colony and inoculate it in 10mL LB liquid medium, 37 ℃, 12h, 150r / min, cultivate...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com