Method for preparing high purity Empagliflozin
An empagliflozin and high-purity technology, which is applied in the field of high-purity empagliflozin preparation, can solve the problems that stubborn impurities cannot be effectively removed, qualified raw materials cannot be achieved, and product purity is low, so as to avoid damage to anhydrous anhydrous Oxygen environment, reduce the number of feeding and detection times, overcome the effect of cumbersome operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
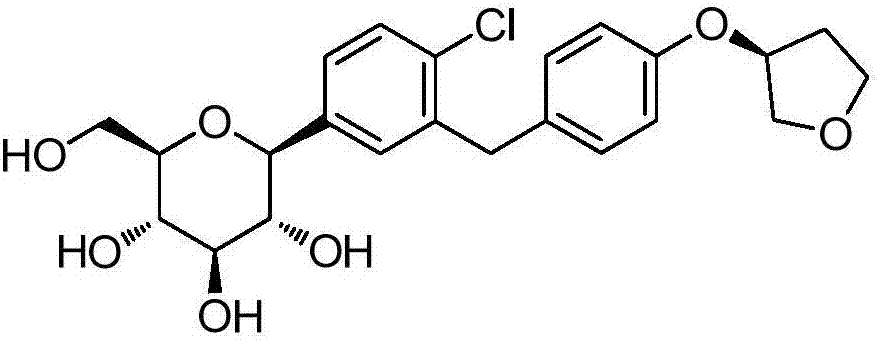
[0029] Example 1 (2S,3R,4S,5R,6R)-2-(4-chloro-3-(4-(((S)-tetrahydrofuran-3-yl)oxy)benzyl)phenyl)-3, Synthesis of 4,5-tris((trimethylsilyl)oxy)-6-(((trimethylsilyl)oxy)methyl)tetrahydro-2H-pyran-2-ol(3)
[0030] Under nitrogen protection, anhydrous tetrahydrofuran (2.0L), compound 1 (1.0kg, 2.4116mol) and compound 2 (1.46kg, 3.1350mol) were added to a 10L glass reaction kettle, stirred and cooled to -30 ℃~-20 ℃, add iPrMgCl / LiCl (2.4L) dropwise to the reaction kettle, control the temperature -30 ℃~-20 ℃, after the reaction HPLC monitoring is completed, the configured 5% citric acid aqueous solution is added dropwise into the reaction kettle, and the addition is finished. After stirring for 30 min, let stand for 30 min, separate the liquid, and collect the organic phase. The organic phase was concentrated under reduced pressure until no condensation dripped out, and the concentrate was collected. After concentration, 2.2 L of n-heptane was added to make a slurry at room tempera...
Embodiment 2
[0031] Example 2 (2S,3R,4S,5R,6R)-2-(4-chloro-3-(4-(((S)-tetrahydrofuran-3-yl)oxy)benzyl)phenyl)-3, Synthesis of 4,5-tris((trimethylsilyl)oxy)-6-(((trimethylsilyl)oxy)methyl)tetrahydro-2H-pyran-2-ol(3)
[0032] Under nitrogen protection, anhydrous tetrahydrofuran (2.0L), compound 1 (1.0kg, 2.4116mol) and compound 2 (1.46kg, 3.1350mol) were added to a 10L glass reaction kettle, stirred and cooled to -30 ℃~-20 ℃, add iPrMgCl / LiCl (2.4L) dropwise to the reactor, control the temperature -30 ℃~-20 ℃, after the reaction HPLC monitoring is completed, the configured 10% citric acid aqueous solution is added dropwise to the reactor, and the addition is completed. After stirring for 30 min, let stand for 30 min, separate the liquid, and collect the organic phase. The organic phase was concentrated under reduced pressure, concentrated until no condensation dripped out, and the concentrate was collected. After concentration, 5L of a mixed solvent of n-heptane and methanol (volume ratio 2...
Embodiment 3
[0033] Example 3 1-C-[4-Chloro-3-[[4-[[(3S)-tetrahydro-3-furyl]oxy]phenyl]methyl]phenyl]-D-glucopyranose glycosides (4)
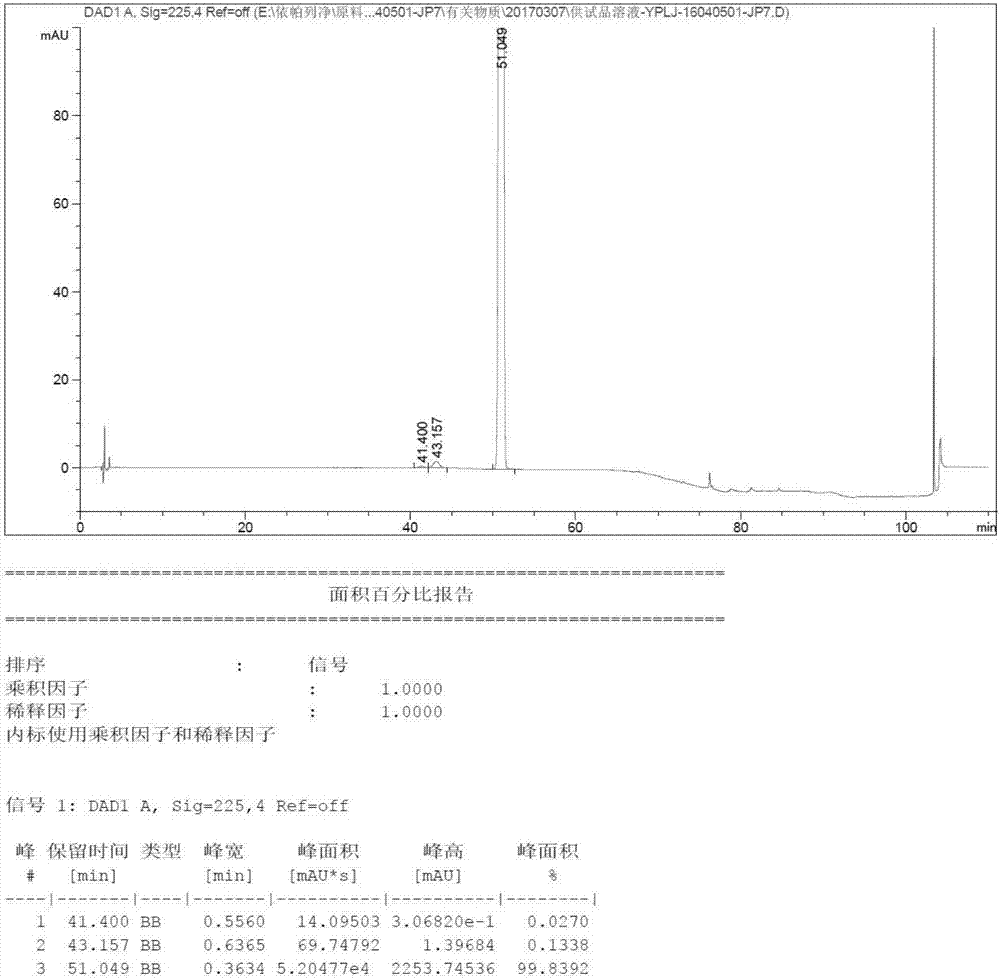
[0034]Add 1.0 kg of (3) prepared in Example 1 or 2 into the reaction kettle, add 2.1 L of methanol, dropwise add concentrated hydrochloric acid, stir to dissolve, and react at room temperature for 4 hours; after the reaction is completed, adjust the pH to Neutral, extract twice with ethyl acetate, combine the organic phases; remove the organic phase by rotary evaporation (40°C ± 5°C), rotate until there is no condensation droplets drop, and obtain a yellow oily liquid with HPLC purity 92.0%. (Agilent liquid phase Chromatography, column with octadecylsilane-bonded silica gel as filler, gradient elution with trifluoroacetic acid aqueous solution / acetonitrile as mobile phase)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com