Lucid ganoderma meroterpenoid compound and medical composite thereof and application thereof
A compound and medicinal salt technology, applied in the field of heteroterpenoids, can solve problems such as drug withdrawal
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] Embodiment 1 prepares the extract of Ganoderma lucidum
[0017] Ganoderma lucidum fruiting bodies were cut into pieces and weighed 2500 grams. Reflux extraction with 10L aqueous solution of 95% ethanol by volume for 3 times, 1 hour each time. The extracts were combined, concentrated and dried under reduced pressure to obtain 170 g of extract.
[0018] The extract was further dissolved with 600 ml of distilled water, extracted three times with an equal volume of n-hexane, and the organic phase was discarded. The aqueous phase was extracted three times with an equal volume of ethyl acetate, the aqueous phase was discarded, and the ethyl acetate extracts were combined. Evaporate ethyl acetate to dryness with a rotary evaporator to obtain 49 g of extract, which is denoted as GS.
Embodiment 2
[0019] The preparation of embodiment 2 compound
[0020] Ganoderma lucidum crude extract ethyl acetate layer GS is separated by silica gel column chromatography, with n-hexane: ethyl acetate system (n-hexane: the volume ratio of ethyl acetate is 1:0, 50:1, 30:1, 20:1, 15:1, 10:1, 4:1, 2:1), dichloromethane: methanol system (the volume ratio of dichloromethane: methanol is 1:0, 100:1, 50:1, 30:1, 10:1 , 5:1) gradient elution was performed sequentially, each gradient was washed with 4 retention volumes, and each retention volume was one fraction. According to the behavioral analysis of thin-layer chromatography, merging of similar fractions obtains fraction GS-1 in normal hexane: ethyl acetate (volume ratio is 1:0) system; in normal hexane: ethyl acetate (volume ratio is 100:1 ~30:1) to obtain fraction GS-2~5; Obtain fraction GS-6, GS-7 in n-hexane: ethyl acetate (volume ratio is 20:1); Obtain fraction GS-6 in n-hexane: ethyl acetate (volume ratio 10:1) to obtain fraction GS-8...
Embodiment 3
[0023] Confirmation of Example 3 Compound 1-3
[0024] NMR, IR, and mass spectrometry were performed on the three prepared compounds to determine the structure of each compound.
[0025] The nuclear magnetic resonance instruments used are Varian Mercury-500 and -600 MHz, the infrared chromatograph is Nicolet IS5FT-IR, and the mass spectrometers are Bruker APEX III 7.0T and APEX II FT-ICR.
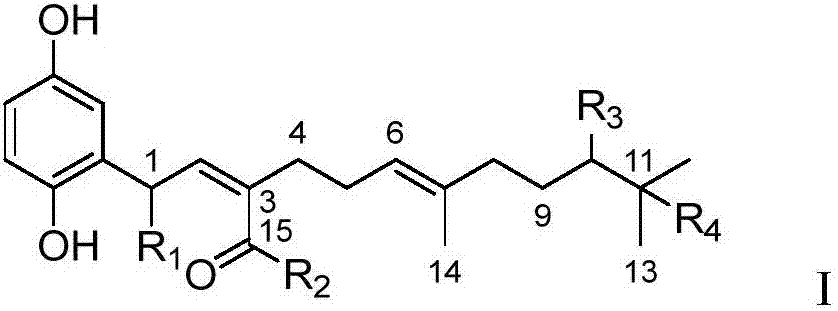
[0026] It was confirmed that compound 1 is a new natural product, which is a farnesyl substituted heteroterpenoid compound containing skeleton I of p-hydroxyphenol.
[0027]
[0028] Compound 1 (Ganomycin J)
[0029] The structural formula of compound 1 is as follows:
[0030]
[0031] (R,2Z,5E)-2-(2-(2,5-Dihydroxyphenyl)ethylidene)-9,10-dihydroxy-6,10-dimethylundec-5-enoic acid
[0032] (R,2Z,5E)-2-(2-(2,5-dihydroxyphenyl)ethylene)-9,10-dihydroxy-6,10-dimethylundec-5-enoic acid
[0033] The carbon spectrum and hydrogen spectrum confirmation data of the NMR of compound 1 are shown...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com