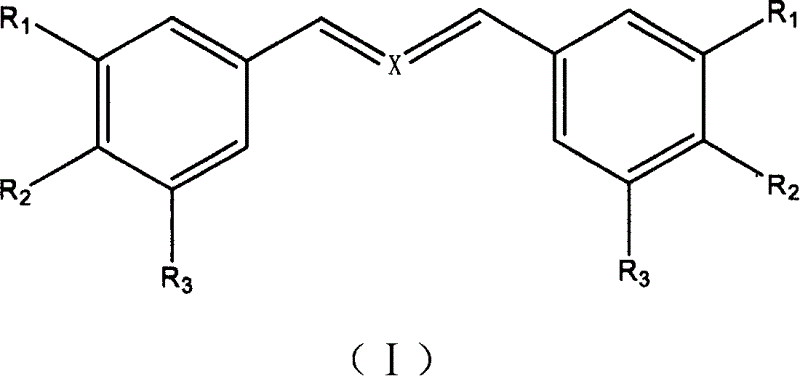
Ramification biphenyl dienones and application
A technology of diphendienone and derivatives, which is applied in the direction of active ingredients of ketones, drug combinations, active ingredients of heterocyclic compounds, etc., to inhibit aldose reductase and non-enzymatic glycosylation, with broad market prospects and economic value high effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
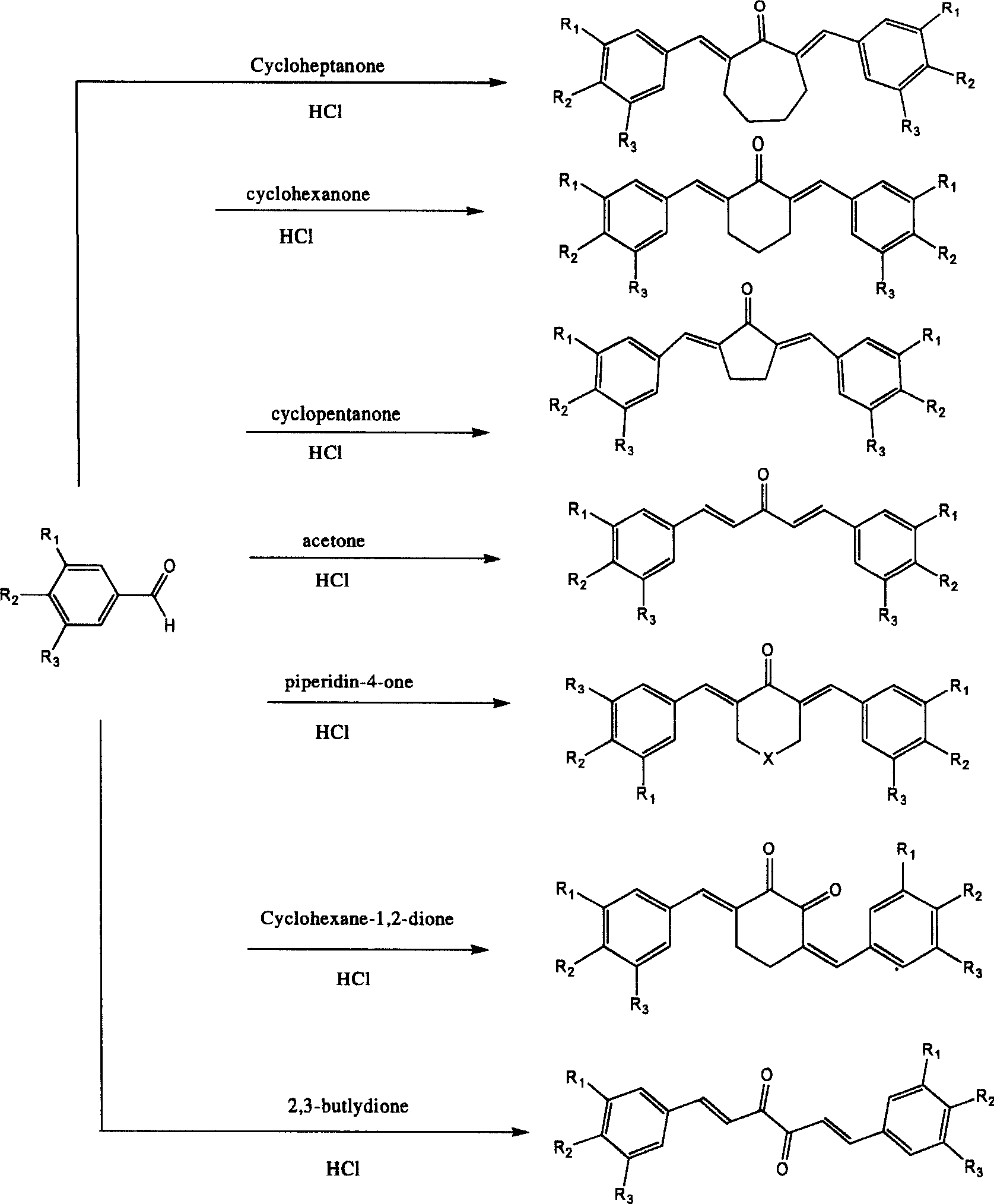
[0025] Example 1 2,7-bis(3-methoxy-4-hydroxybenzylidene) cycloheptanone (I 4 )Synthesis
[0026] Add 15.0g vanillin (0.1mol) and 5.5mL (0.05mol) cycloheptanone into the round bottom flask, stir at room temperature (20-25°C), add 2.0ml concentrated hydrochloric acid dropwise after the mixture is homogeneous, and continue stirring for 2 Leave it for 3 days after hours. The reaction mixture was treated with 1:1 aqueous acetic acid and filtered. Rinse with ethanol and hot water, dry in vacuo, and recrystallize from ethanol to obtain compound I 4 , the yield was 82%.
[0027]
[0028] Compound I 4
Embodiment 2
[0029] Example 2 2,6-bis(3,4-dihydroxybenzylidene)cyclohexanone (II 2 )Synthesis
[0030] Add 12.2g of 3,4-dihydroxybenzaldehyde (0.1mol) and 5.0mL (0.05mol) of cyclohexanone into the round bottom flask, add 20ml of saturated hydrochloric acid acetic acid solution, stir at room temperature (20-25°C), stir for 2 After 2 hours, stand for 2 days and filter. Rinse with ethanol and hot water, and dry in vacuo to obtain compound II 2 , the yield was 90%.
[0031]
[0032] Compound II 2
Embodiment 3
[0033] Example 3 2,5-bis(4-hydroxybenzylidene)cyclopentanone (III 1 )Synthesis
[0034] Add 15.0g vanillin (0.1mol) and 4.5mL (0.05mol) cyclopentanone into the round bottom flask, stir at room temperature (20-25°C), add 2.0ml concentrated hydrochloric acid dropwise after the mixture is homogeneous, and continue stirring for 2 Leave it for 3 days after hours. The reaction mixture was treated with 1:1 aqueous acetic acid and filtered. Rinse with ethanol and hot water and dry under vacuum. Compound III was obtained by recrystallization from methanol 1 , the yield was 90%.
[0035]
[0036] Compound III 1
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com