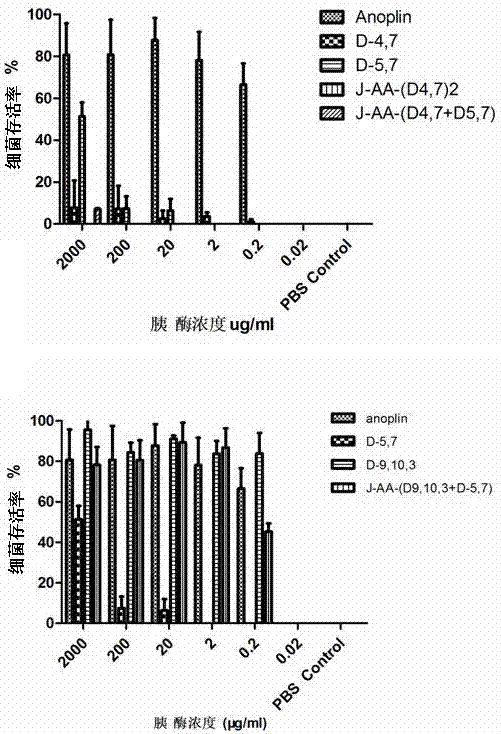
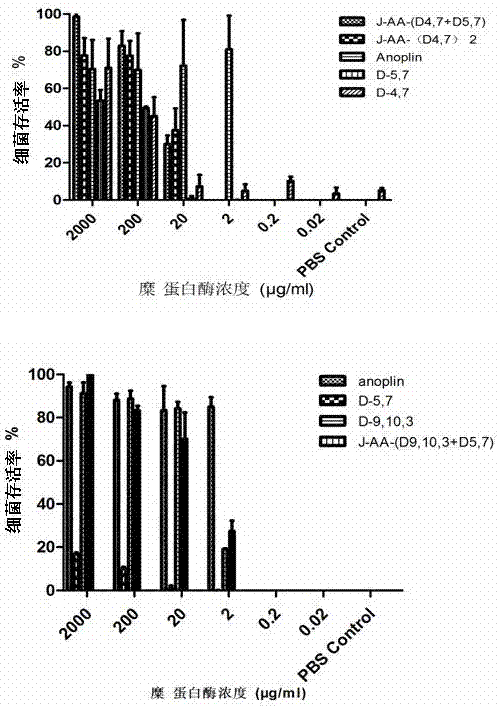
Antibacterial peptide dimer analogues containing D type amino acids as well as synthesis and application of dimer analogues
A peptide dimer and amino acid technology, applied in the field of biochemistry, can solve the problems of reduced antibacterial activity and no improvement of antibacterial activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Embodiment 1, the synthesis of analogue J-AA-(D4,7) 2
[0044] Weigh Rink-MBHA resin, the substitution value is 0.43mmol / g, and the amount of peptide is 0.15mmol, add redistilled dichloromethane solution, fully stir and swell the resin for 30min, then add 10% hexahydropyridine to remove the resin Fmoc protection, wash 2 minutes each time, wash 4 times in total, then wash the resin with redistilled DMF solution, the same 2 minutes each time, wash 4 times in total, and finally use indene detection indicator (phenol: potassium cyanide pyridine: ninhydrin = 1:2:1) To check whether the resin has removed the Fmoc protecting group, boil the resin for 1 minute, observe immediately, and the resin turns blue, which proves that the Fmoc group has been removed. According to the sequence of the peptide, connect the amino acid from the C-terminus, Prepared by RP-HPLC and purified by C18 column, the elution condition is 20%~60% acetonitrile, 8ml / min, and the precursor peptides Ac-Pra-...
Embodiment 2
[0045] Embodiment 2, the synthesis of analogue J-AA-(D4,7+D5,7)
[0046] Weigh Rink-MBHA resin, the substitution value is 0.43mmol / g, and the amount of peptide is 0.25mmol, add redistilled dichloromethane solution, fully stir and swell the resin for 30min, then add 15% hexahydropyridine to remove the resin Fmoc protection, wash for 2 minutes each time, wash 4 times in total, then wash the resin with redistilled DMF solution, the same 2 minutes each time, wash 4 times in total, and then detect the indicator (phenol: potassium cyanide pyridine: ninhydrin = 1 :2:1) test, the resin is blue, which proves that the Fmoc group on the resin has been removed. According to the sequence of the peptide, amino acids are connected from the C-terminus, prepared by RP-HPLC, and purified using a C18 column. The elution condition is: 20%-60% acetonitrile, 8ml / min purification, to get the precursor peptide Ac-Pra-D4,7 and Ac-Lys(N3)-D5,7. The obtained precursor peptides were subjected to click c...
Embodiment 3
[0047] Embodiment 3, the synthesis of analogue J-AA-(D9,10,3+D5,7)
[0048] Weigh the Rink-MBHA resin, the substitution value is 0.43mmol / g, the amount of peptide synthesis is 0.2mmol, add redistilled dichloromethane solution, fully stir and swell the resin for 30min, then add 15% hexahydropyridine to remove the resin Fmoc protection, wash 2 minutes each time, wash 4 times in total, then wash the resin with redistilled DMF solution, the same 2 minutes each time, wash 4 times in total, and then detect the indicator (phenol: potassium cyanide pyridine: ninhydrin = 1 :2:1) test, the resin is blue, which proves that the Fmoc group has been removed. According to the sequence of the peptide, amino acids are connected from the C-terminus, prepared by RP-HPLC, and purified using a C18 column, and the elution condition is 20%- 60% acetonitrile, 8ml / min purification, to obtain the precursor peptide Ac-Pra-D9,10,3 and Ac-Lys(N3)-D5,7. The obtained precursor peptides were subjected to cl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com