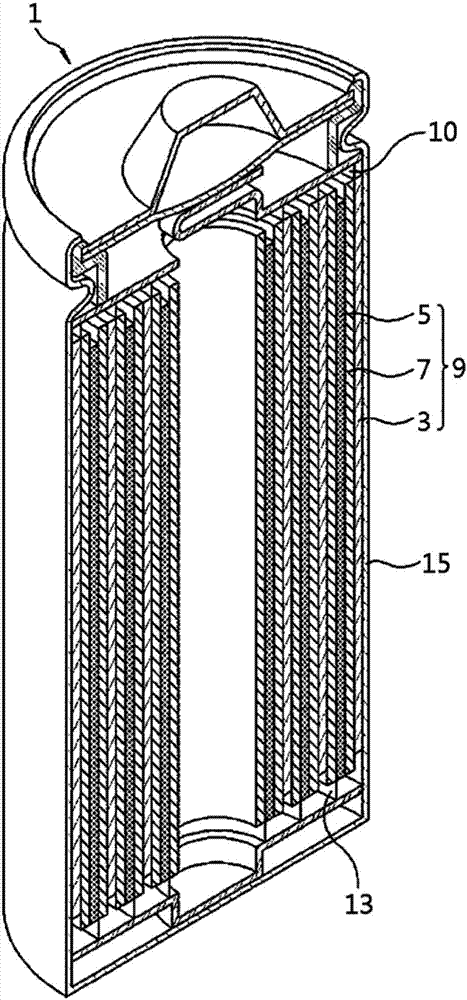
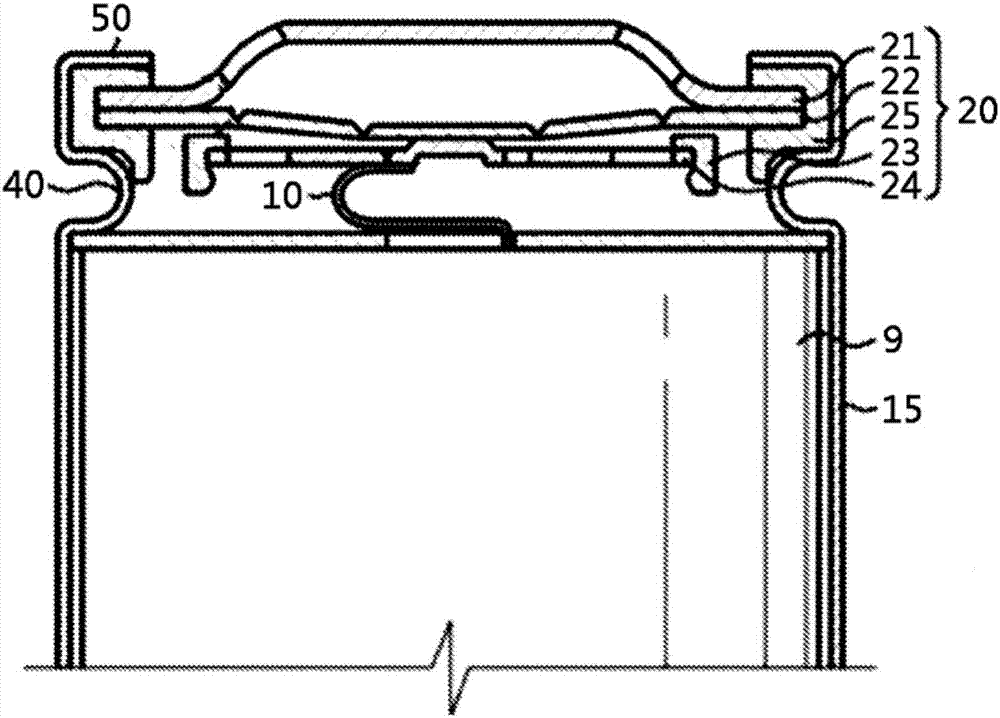
Electrochemical device
An electrochemical and current interrupt device technology, applied in electrochemical generators, batteries, circuits, etc., can solve the problems of accelerating capacity, increasing side reactions, deterioration, etc., to achieve the effect of accelerating capacity deterioration and increasing side reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0107] A composition for forming an anode active material layer is prepared by mixing graphite, carbon black conductive material, and PVdF binder in N-methylpyrrolidone solvent, and then applying the composition to a copper current collector to form Negative electrode active material layer.
[0108] A composition for forming a positive electrode active material layer prepared by mixing LNMO positive electrode active material, carbon black conductive material, and PVdF binder in an N-methylpyrrolidone solvent, and then applying the composition to an aluminum current collector On, a positive electrode active material layer is formed.
[0109] An electrode assembly was prepared by inserting a porous polyethylene separator between the positive electrode and graphite negative electrode prepared as described above, and by placing the electrode assembly in a case and injecting an electrolyte until the available space volume (EV) relative to the case A lithium secondary battery was p...
experiment Embodiment 1
[0113] (Experimental Example 1: Detect the physical properties of the prepared secondary battery)
[0114] The lithium secondary battery prepared in this example has an available space volume (EV) of 20% by volume relative to the total volume of the cavity (CV) in the case, and 80% by volume, and when the lithium secondary battery is charged at 1C and discharged at 1C at 25°C, and the charge and discharge are taken as one cycle, and the cycle is repeated 100 times, at 25°C and 1kgf / cm 2 Under certain conditions, the volume (GV) occupied by the gas generated in the lithium secondary battery is 6 times the available space volume (EV), and the pressure in the casing is 6kgf / cm 2 .
[0115] The lithium secondary battery prepared in the above comparative example has an available space volume (EV) of 46% by volume relative to the total cavity volume (CV) in the case, and an 56% by volume, and in the state where the lithium secondary battery is charged at 1C and discharged at 1C at...
experiment Embodiment 2
[0116] (Experimental Example 2: Detection of Lifetime Characteristics)
[0117] The battery life characteristics of the lithium secondary batteries prepared in the above Examples and Comparative Examples were examined. Under the condition of 1C / 1C charge / discharge at 25°C, 100 charge and discharge cycles were carried out, and two measurements were carried out for each case. The results are listed in Figure 4 . exist Figure 5 Among them, the examples show the case of a large amount of electrolytic solution, and the comparative examples show the case of a small amount of electrolytic solution.
[0118] refer to Figure 5 , it can be seen that the lithium secondary battery manufactured in this example has reduced capacity degradation compared to the lithium secondary battery prepared in Comparative Example, thereby improving life characteristics.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com