Method for producing ketone and/or alcohol, and system thereof
一种制造方法、制造系统的技术,应用在酮和/或醇的制造及其系统领域,能够解决分解选择率低、氢过氧化物分解速度慢、不利等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
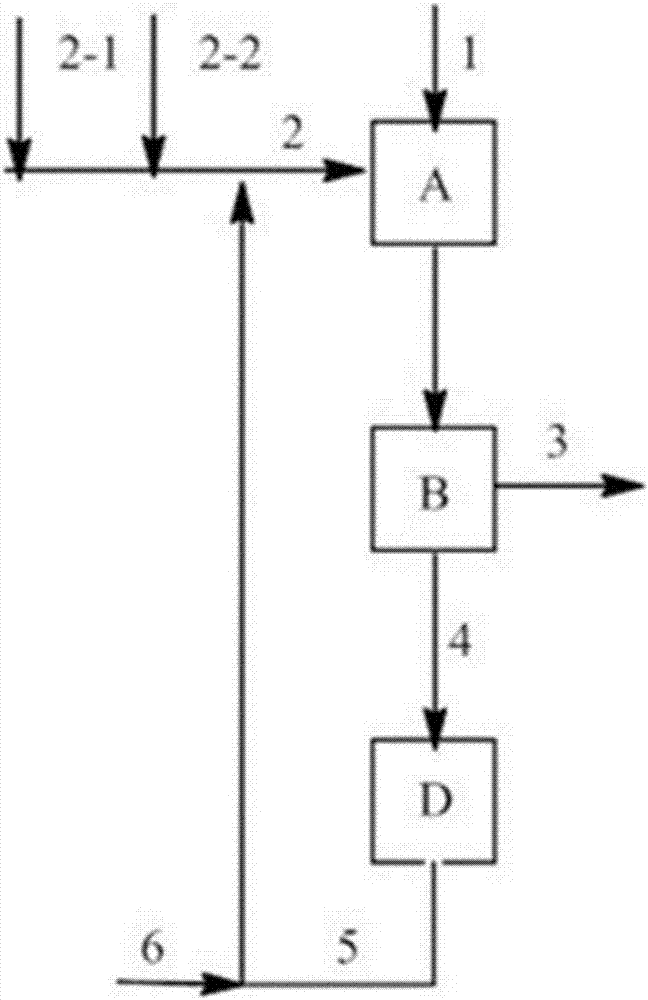
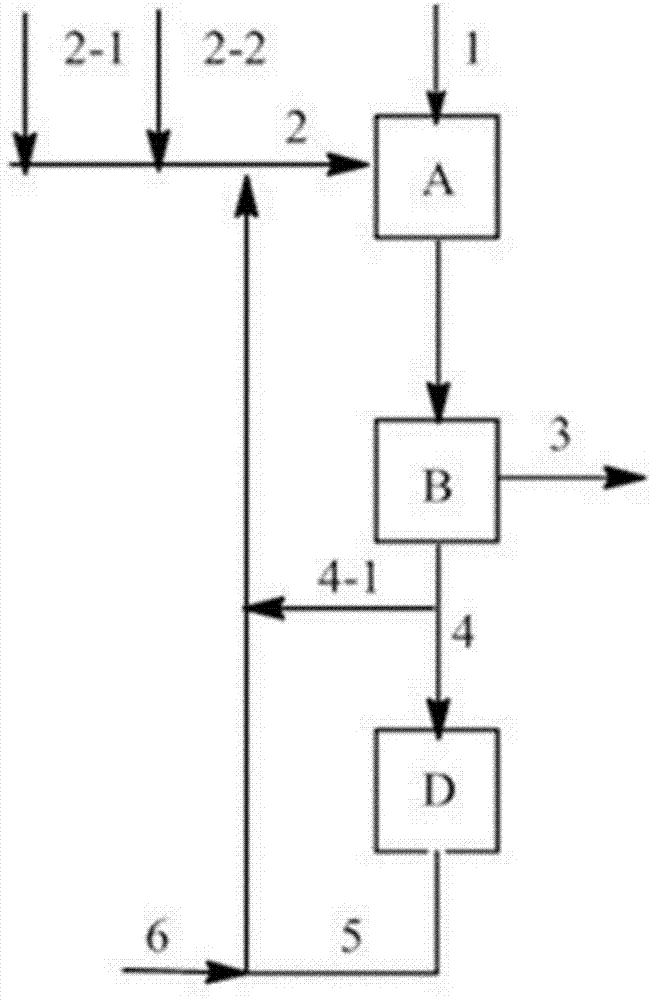
Image
Examples
Embodiment 1
[0112] 450 g of cyclohexane and 40 g of a 17% by weight aqueous solution of sodium carbonate containing 10 wt. ppm of Co (cobalt) were added to a reactor made of SUS with an internal volume of 500 ml coated with Teflon (registered trademark) on the surface, and heated. to 125°C. In this mixed solution, 50 g of a cyclohexane solution containing 20% by weight of cyclohexyl hydroperoxide, 10% by weight of cyclohexanone, and 20% by weight of cyclohexanol was injected, and samples were collected at regular intervals. While measuring the decomposition rate of cyclohexyl hydroperoxide. The overall decomposition rate constant of cyclohexyl hydroperoxide was 0.063 (1 / min), and the decomposition rate after 20 minutes was 78.4%. In addition, the pH of the aqueous phase before the reaction was 11.9, and the pH of the aqueous phase after the reaction was 10.7.
Embodiment 2
[0114] The reaction was carried out in the same manner as in Example 1 except that the water phase charged into the reactor made of SUS contained 10 weight ppm of Co, 17 weight % of sodium carbonate, and 2.5 weight % of valeric acid. The overall decomposition rate constant of cyclohexyl hydroperoxide was 0.226 (l / min), and the decomposition rate after 20 minutes was 99.7%. In addition, the pH of the aqueous phase before the reaction was 10.3, and the pH of the aqueous phase after the reaction was 10.0.
Embodiment 3
[0116] The reaction was carried out in the same manner as in Example 1, except that the water phase charged into the reactor made of SUS contained 10 weight ppm of Co, 17 weight % of sodium carbonate, and 2.5 weight % of hexanoic acid. The overall decomposition rate constant of cyclohexyl hydroperoxide was 0.199 (l / min), and the decomposition rate after 20 minutes was 99.1%. In addition, the pH of the aqueous phase before the reaction was 10.3, and the pH of the aqueous phase after the reaction was 10.0.
PUM
| Property | Measurement | Unit |
|---|---|---|
| surface pH | aaaaa | aaaaa |
| surface pH | aaaaa | aaaaa |
| surface pH | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com