Trichina 7TR protein human single-chain antibody and preparation method and medical application
A single-chain antibody and trichinella technology, applied in the field of bioengineering, can solve problems such as difficult large-scale production and application, limited therapeutic effect, and high cost of mouse-derived antibodies
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
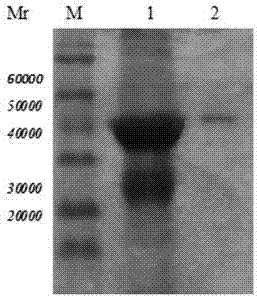
[0038] Purification and renaturation of Trichinella spiralis 7TR protein
[0039] Inoculate recombinant bacteria BL21 / pET32a-7TR into TB medium, induce expression with IPTG, collect the bacteria by centrifugation, and centrifuge after sonication, discard the supernatant. After the precipitate was purified by urea gradient, the crude and pure Trichinella spiralis 7TR recombinant protein was obtained. Take 20ml of the crude and pure Trichinella spiralis 7TR fusion protein solution, and put it in the dialysate (20mM Na 2 HPO 4 , 6M Urea pH=8.0) dialyzed overnight. The dialyzed solution was purified by DEAE anion exchange chromatography, liquid A: 20mM Na 2 HPO 4 , 6M Urea (pH=8.0) equilibrates the chromatography column, loads the sample, washes and flows through, 0%-100% B is refolded on the gradient column (B: 20mM Na 2 HPO 4, pH=8.0), during which the total liquid flow is 500ml. After reaching 100% B, use the eluent (20mM Na 2 HPO 4, 0.35M NaCl) to collect the target p...
Embodiment 2
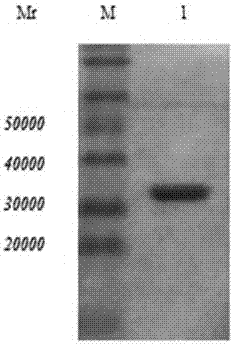
[0041] Screening of Single Chain Antibody to Trichinella spiralis 7TR Protein
[0042]After preparing the secondary phage antibody library, the purified Trichinella spiralis 7TR protein was diluted with PBS, coated on a 96-well microtiter plate, 1 μg per well, overnight at 4°C. Discard the supernatant the next day, wash 3 times with PBS, then block with 200 μL 5% Milk-PBS at 37°C for 2 hours, discard the blocking solution and wash with PBS. Take 1 μl of the secondary antibody library and dilute it with 2% Milk-PBS, 100 μl per well, shake vigorously at room temperature for 60 minutes, let stand for 60 minutes, then discard the liquid, wash with PBST 10 times, dry the residual liquid after washing, and add 50 μl to each well The trypsin eluate was shaken vigorously for 15 minutes at room temperature to elute the phage. Add 1.7 mL of freshly recovered E.coli TG1 prepared in advance to 300 μl of phage eluent, mix well, and let stand in a water bath at 37°C for 30 minutes. Dilute...
Embodiment 3
[0047] Validation of A549 binding activity of Trichinella spiralis 7TR single chain antibody
[0048] A549 cells were digested with 5‰ trypsin to make a single-cell suspension, and the cells were counted and cell slides were carried out. Place the 24-well cell culture plate at 37°C in 5% CO 2 After culturing in the cell incubator for 24 hours, the culture medium was sucked out, and the slides were washed 3 times with 37°C preheated PBS, 5 minutes each time. Fix with 4% paraformaldehyde for 30 min, wash with PBS and dry the slides. Wash the dried slides with water, punch with 1‰ TritonX-100 for 10 min, wash with PBS, and use 3% H 2 o 2 Block for 30 minutes, wash with PBS as before, add 5% PBS-Milk to block. Discard the PBS-Milk used for blocking, and add the primary antibody diluted 1:50 on the glass plate. overnight at 4°C. Wash with PBS as before, add secondary antibody (Protein A full length protein-FITC) and wash with PBS. Add DAPI nuclear staining reagent and incuba...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com