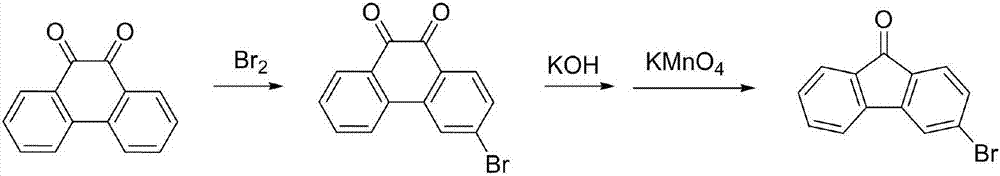
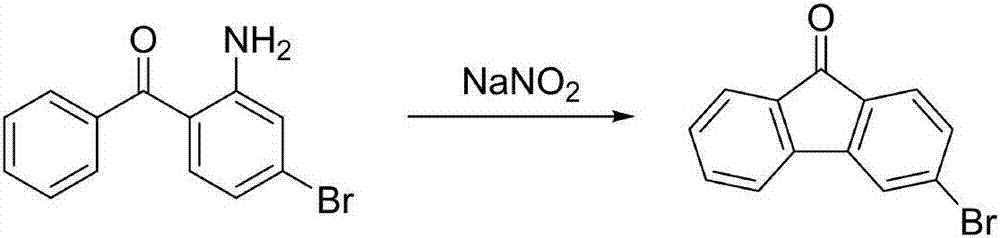
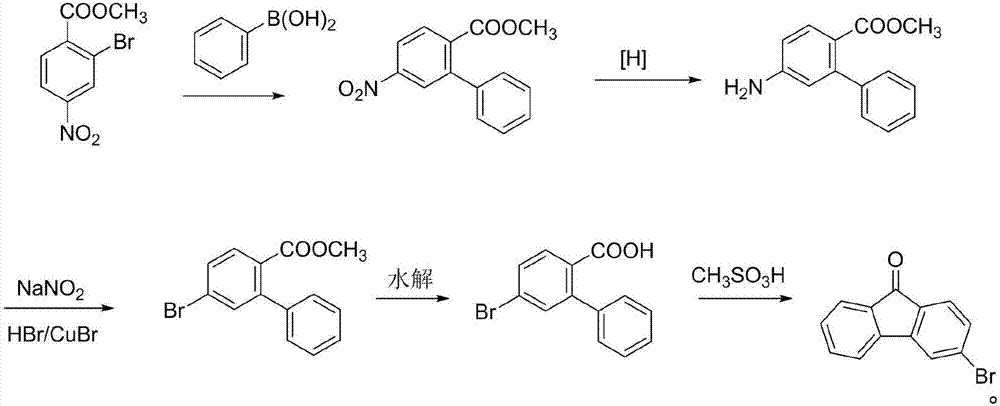
3-bromofluorenone preparation method
A technology of bromofluorenone and fluorenone, which is applied in the field of preparation of 3-bromofluorenone, can solve the problems of difficult to meet organic optoelectronic materials, difficulty in product purification, long reaction route, etc., and achieve broad market prospects, low cost, and preparation The effect of simple method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Preparation of fluorenone methoxyxime: Dissolve 36.0g (200mmol) fluorenone, 17.5g (210mmol) methoxyamine hydrochloride and 13g (122mmol) sodium carbonate in 250mL methanol solution, heat up to 60-65°C, and stir for reaction 3.0hrs. Cool down to room temperature, add 100g and 200g chloroform to the reaction system, separate layers, wash the organic phase with 100mL of water, dry over anhydrous sodium sulfate, and remove the solvent in vacuo to obtain 41.6g of crude fluorenone methoxyxime, with a yield of 99.52%, without purification, directly for the next step.
[0034] Preparation of 3-bromofluorenone methoxyxime: 21.0g (100mmol) fluorenone methoxyxime and 1g (7.5mmol) aluminum trichloride were added to 200g dichloroethane, fully replaced by nitrogen, at 50 C bromine dichloroethane mixture (15.9g (100mmol) bromine + 100g dichloroethane) was added dropwise. The dropwise addition took about 1 hour. After the reaction was completed, 100mL of saturated aqueous sodium bisu...
Embodiment 2
[0043] Preparation of fluorenone methoxyxime: Dissolve 36.0g (200mmol) fluorenone, 16.7g (200mmol) methoxyamine hydrochloride and 11.7g (110mmol) sodium carbonate in 250mL methanol solution, heat up to 60-65°C, stir Response 3.0hrs. Cool down to room temperature, add 100g and 200g chloroform to the reaction system, separate layers, wash the organic phase with 100mL of water, dry over anhydrous sodium sulfate, and remove the solvent in vacuo to obtain 41.4g of crude fluorenone methoxyxime, with a yield of 99.04%, without purification, directly for the next step.
[0044] Preparation of 3-bromofluorenone methoxyxime: 21.0g (100mmol) fluorenone methoxyxime and 0.7g (5mmol) aluminum trichloride were added to 200g dichloroethane, fully replaced by nitrogen, at 60 C bromine dichloroethane mixture (14.3g (90mmol) bromine + 100g dichloroethane) was added dropwise. The dropwise addition took about 1 hour. After the reaction was completed, 100mL of saturated aqueous sodium bisulfite s...
Embodiment 3
[0053] Preparation of fluorenone methoxyxime: Dissolve 36.0g (200mmol) fluorenone, 18.4g (220mmol) methoxyamine hydrochloride and 13g (122mmol) sodium carbonate in 250mL ethanol solution, heat up to 70-75°C, and stir for reaction 3.0hrs. Cool down to room temperature, add 100g and 200g chloroform to the reaction system, separate layers, wash the organic phase with 100mL of water, dry over anhydrous sodium sulfate, and remove the solvent in vacuo to obtain 41.8g of crude fluorenone methoxyxime, with a yield of 100%, without purification, directly for the next step.
[0054] Preparation of 3-bromofluorenone methoxyxime: 21.0g (100mmol) fluorenone methoxyxime and 1.3g (10mmol) aluminum trichloride were added to 200g dichloroethane, fully replaced by nitrogen, at 40 C bromine dichloroethane mixture (15.2g (95mmol) bromine + 100g dichloroethane) was added dropwise. The dropwise addition took about 1 hour. After the reaction was completed, 100mL of saturated aqueous sodium bisulfi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com