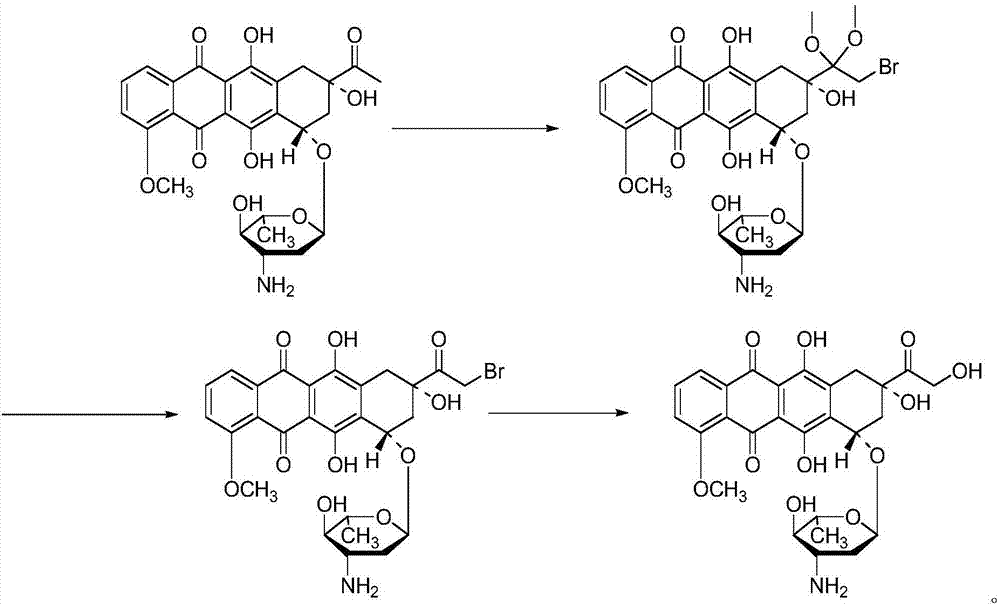
Epirubicin hydrochloride intermediate compound IV
A technology of epirubicin hydrochloride and compound, applied in the direction of sugar derivatives, sugar derivatives, organic chemistry, etc., can solve the problems of high technical requirements, serious environmental pollution, easy decomposition, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0113] Preparation of intermediate compound III:
[0114]Add dichloromethane and daunorubicin hydrochloride (daunorubicin hydrochloride:dichloromethane=1:60 (W / V, g / mL)) into a 1000 mL three-necked glass reaction flask. Start stirring, control the temperature at 5°C, and check the dissolution of the material after 10 to 15 minutes. If it is not completely dissolved, continue to stir; Daunorubicin hydrochloride: methyl alcohol: camphorsulfonic acid=1:177:1 (W / V / W, g / mL / g)), after stirring for 1 hour, add trimethyl orthoformate (daunorubicin hydrochloride: Trimethyl orthoformate=1:2 (W / V, g / mL)); after adding, stir for 2 hours; Element: trifluoroacetic anhydride = 1:2 (W / V, g / mL), after the addition is complete, stir for 1 hour.
[0115] After completion of the reaction, methanol (daunorubicin hydrochloride: methanol=1:30 (W / V, g / mL)) was added to the reaction solution, and after stirring for 10 minutes, 8% sodium bicarbonate solution was added to adjust the pH value to 7.0~ ...
Embodiment 2
[0117] Preparation of intermediate compound III:
[0118] Add 1,4-dioxane and daunorubicin hydrochloride (daunorubicin hydrochloride: 1,4-dioxane=1:70 (W / V, g / mL) in the 1000mL three-neck glass reaction flask )). Start stirring, control the temperature at 10°C, and after 10 to 15 minutes, check the dissolution of the material, if it is not completely dissolved, continue stirring; if it is completely dissolved, cool the material liquid to 5°C, and add a methanol solution of camphorsulfonic acid ( Daunorubicin hydrochloride: methyl alcohol: camphorsulfonic acid=1:177:1 (W / V / W, g / mL / g)), after stirring for 1 hour, add trimethyl orthoformate (daunorubicin hydrochloride: Trimethyl orthoformate=1:1 (W / V, g / mL)); after adding, stir for 3 hours; Vegetation: trifluoroacetic anhydride = 1:4 (W / V, g / mL)), after the addition was complete, stir for 1.5 hours.
[0119] After completion of the reaction, add methanol (daunorubicin hydrochloride: methanol=1:40 (W / V, g / mL)) in the reaction s...
Embodiment 3
[0121] Preparation of intermediate compound III:
[0122] Add tetrahydrofuran and daunorubicin hydrochloride (daunorubicin hydrochloride: tetrahydrofuran = 1:50 (W / V, g / mL)) into a 1000 mL three-necked glass reaction flask. Start stirring, control the temperature at 8°C, and check the dissolution of the material after 10 to 15 minutes. If it is not completely dissolved, continue to stir; Daunorubicin hydrochloride: methyl alcohol: camphorsulfonic acid=1:177:1 (W / V / W, g / mL / g)), after stirring for 1 hour, add trimethyl orthoformate (daunorubicin hydrochloride: Trimethyl orthoformate=1:5 (W / V, g / mL)); after adding, stir for 3 hours; Element: trifluoroacetic anhydride = 1:5 (W / V, g / mL)), after the addition was complete, stir for 1.5 hours.
[0123] After completion of the reaction, add methanol (daunorubicin hydrochloride: methanol=1:20 (W / V, g / mL)) in the reaction solution, after stirring for 15 minutes, add 8% sodium bicarbonate solution to adjust the pH value to 7.0~ 8.0. T...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com